Purpose: SuperSonic Imagine (SSI) measure the tissue elasticity in real-time[1]. The goal of this study was to characterize in a canine model of aneurysm endovascular repair (EVAR) residual endoleak[2] and thrombus organization[3] with SSI after endoleak embolization and correlate results with CT-Scan, Doppler Ultrasound (DUS) and pathologic findings.
Materials and Methods: EVAR was done with creation of type I endoleak in eighteen aneurysms created in nine dogs (common iliacs arteries)[4]. Two embolization gels (Chitosan (Chi) or Chitosan-Sodium-Tetradecyl-Sulfate (Chi-STS)) were injected in the sac to seal the endoleak and promote healing[5]. SSI and DUS were performed at baseline (implantation, 1-week, 1-month, 3-months) whereas angiography and CT-scan were performed at sacrifice. Macroscopic and histopathological analyses were processed to identify and segment five different regions of interest (ROIs) (endoleak, fresh or organized thrombus, Chi or Chi-STS). Elasticity modulus values were compared.
Results: At sacrifice, 10 aneurysms had type I endoleaks, 9 had fresh thrombus, 15 had organized thrombus and 3 were completely sealed. SSI was able to detect all endoleaks seen on CT-scan and macroscopic cuts. We found elastic moduli (in kPa) of 0.1 ± 0.2, 9.2 ± 3.5, 47.3 ± 25.7, 55.9 ± 21.7 and 69.6 ± 29.0 in endoleak, fresh thrombus, organized thrombus, Chi and Chi-STS regions at 3 months, respectively. Elasticity values of endoleak and fresh thrombus were significantly lower than others ROIs (p < 0.001). Figure 1 displays an AAA with different ROIs visualized with SSI. Figure 2 shows an aneurysm with massive fresh thrombus detected as an area of lower stiffness. Elasticity values of fresh thrombus ranged between 3 and 19 kPa (8.7 ± 3.6 kPa) at 1-week and 30.2 ± 13.8 kPa at 3-months indicating that SSI can evaluate thrombus maturation.

Figure 1. Images from an aneurysm with endoleak (green arrow), chitosan (white arrow), chitosan less organized (yellow arrow) and organized thrombus (black arrow). Endoleak and fresh thrombus were visualized on SSI. Areas filled with Chi were slightly hyper echoic and with endoleak were anechoic on B-mode US. (A) Macroscopic cut. (B) CT-scan. (C) DUS. (D) B-mode US. (E) SSI. (F) Color scale values for SSI.
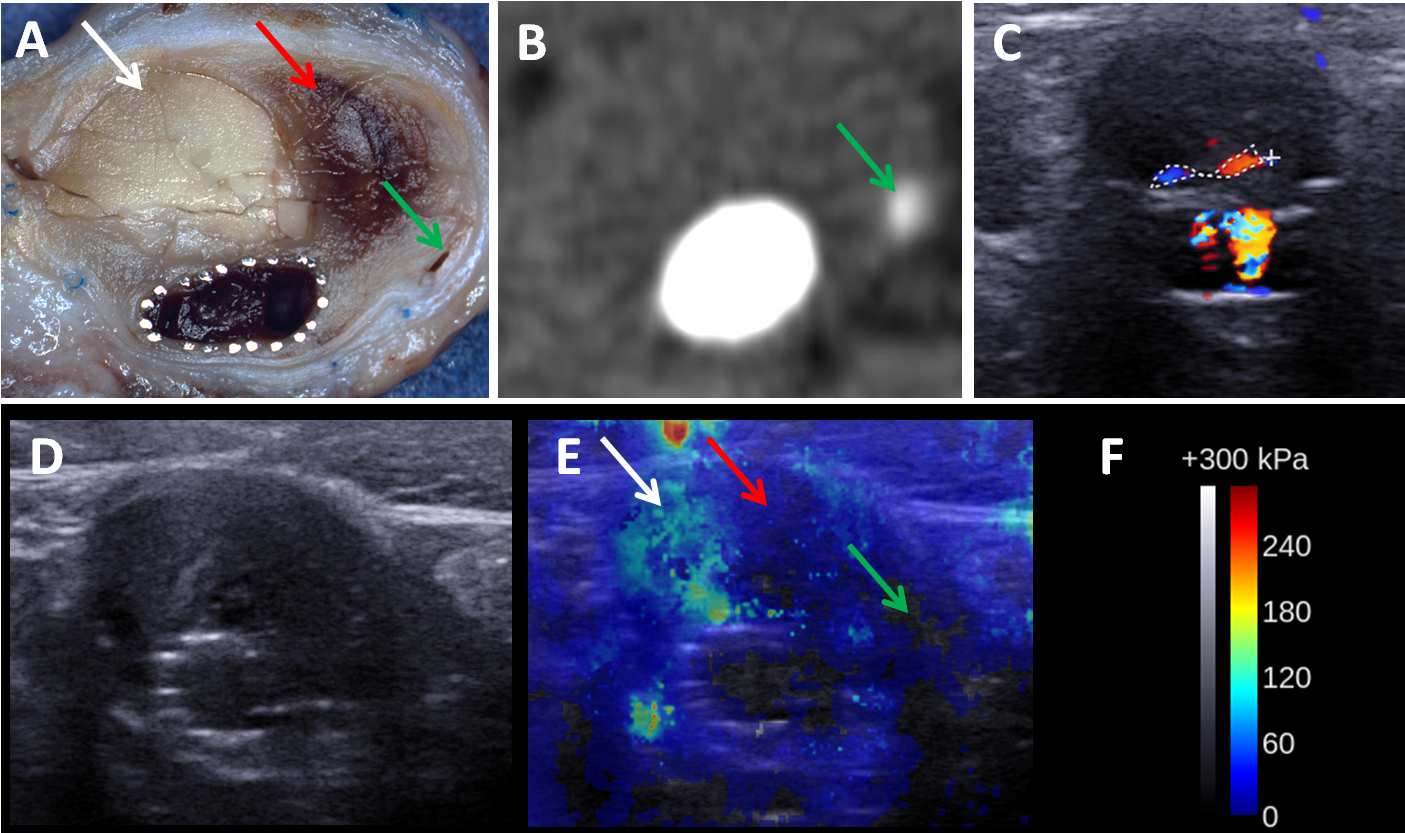
Figure 2. Axial views of the different techniques from an aneurysm with massive fresh thrombus and a small endoleak saw on SSI. (A) Macroscopic cut. (B) CT-scan. (C) DUS. (D) B-mode US. (E) SSI. (F) Color scale values for SSI. The green, red and white arrows indicate endoleak, fresh thrombus and chitosan, respectively.
Conclusion: The results confirm that SSI was able to evaluate thrombus organization and embolization agents over time after endoleak embolization following EVAR. A lower elastic modulus value corresponds to fresh thrombus wheres a higher value corresponds to organized thrombus.
Clinical Relevance: The SSI can complement conventional DUS in post-EVAR surveillance. It could reduce the cost, the exposition to ionizing radiation and nephrotoxic contrast agents of surveillance CT-scan follow-up.
Fonds de Recherche du Québec en Santé (FRQS); Canadian Institutes of Health Research
References:
[1] Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435.
[2] White GH, Yu W, May J, et al. Endoleaks as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg 1997;4:152-68.
[3] White GH. What are the causes of endotension? J Endovasc Ther 2001;8:454-6.
[4] Lerouge S, Raymond J, Salazkin I, Qin Z, Gaboury L, Cloutier G, et al. Endovascular aortic aneurysm repair with stent-grafts: experimental models can reproduce endoleaks. J Vasc Interv Radiol 2004;15:971–9.
[5] Fatimi A, Chabrot P, Berrahmoune S, Coutu JM, Soulez G, Lerouge S. A new injectable radiopaque chitosan-based sclerosing embolizing hydrogel for endovascular therapies. Acta Biomater. 2012 Jul;8(7):2712-21.