Introduction: Encapsulation within alginate microcapsules is being actively developed for use in islet transplantation due to its advantages of safety, easy delivery and elimination of immunosuppressive therapy requirements. It is believed that alginate microcapsules exhibit isotropic shrinking at high culture temperatures and isotropic swelling in the presence of sodium ions. In this study, we evaluated various gelling ions and alginates to study their responses at specific temperatures and at physiological sodium ion concentrations to develop strategies to mitigate sodium ion-induced isotropic swelling of the alginate capsules.
Materials and Methods: Aqueous solutions of ultra-pure low viscosity mannuronate (UP LVM, NovaMatrix® PRONOVA™) and ultra-pure low viscosity gluronate (UP LVG) at 2.5% w/v were used to generate alginate microcapsules using a compressed air-driven electrostatic encapsulator (Nisco Engineering AG). Standard settings of 4-5 psi (pressure), 80 rpm (agitator speed), 30 mm (needle height) and 25G (needle height) were used. Three different divalent ion solutions were compared: 20mM BaCl2, 50mM BaCl2, and 120mM CaCl2 (n=3, performed in triplicate). After each experiment, a minimum 100 microcapsules were imaged using an inverted bright field microscope after 15 min of crosslinking. Microcapsules were then stored in a buffered solution containing proteins and ions at physiological concentrations or in a 5mM buffer of CaCl2 or BaCl2 depending on the ion used for polymerization. The microcapsules were then either stored at either 3oC or 37oC. After 24 hrs, 72 hrs, 7 days, 11 days and 14 days of incubation, groups of 100 microcapsules were quantified for isotropic changes in diameter. Alginate capsules were then transferred to the following solutions:
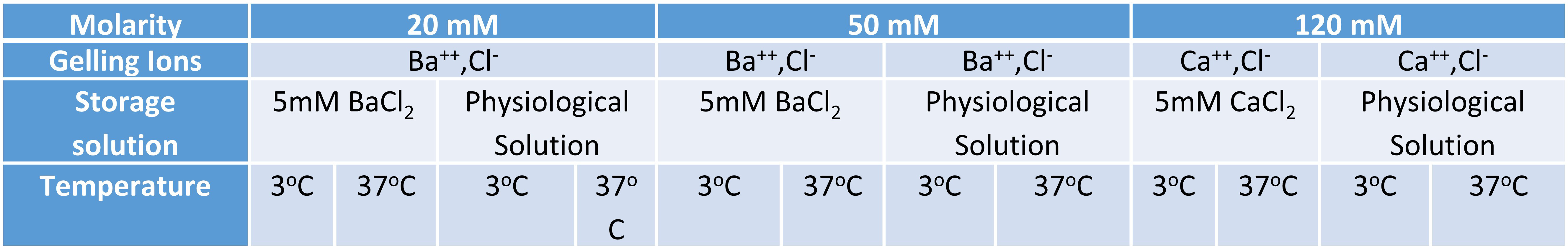
Images obtained as described were processed using a batch-processing algorithm on Image J (NIH). Microcapsule size was analyzed and compared. All results are expressed as Mean±SEM. A Mann-Whitney Test was performed to analyze whether the change in microcapsule size was statistically significant (p<0.05).
Results: At the end of the study period, both UP LVG and UP LVM alginate microcapsules exhibited significant isotropic shrinking at 37oC (-4.2±0.2%, UP LVG; -6.1±0.1%, UP LVM) compared to those incubated at 3oC (p=0.04, ANOVA). Barium-gelled alginate microcapsules demonstrated isotropic shrinking regardless of alginate type, gelling ion and incubation conditions (-6.1±0.2%, UP LVG; -6.6±0.1%, UP LVM). Calcium-gelled UP LVM microcapsules demonstrated significant isotropic swelling when incubated in the physiological solution (+5.5±0.2%) compared to all other groups (p<0.01, ANOVA).
Conclusions: The effects of alginate type, and choice of gelling ion directly influence microcapsule size during in vitro incubation in physiological solutions. Since microcapsules are being evaluated for use in transplantation studies, it would be pertinent to develop strategies to further evaluate barium-gelled alginate UP LVM and UP LVG microcapsules with in vivo studies to evaluate their safety and biocompatibility for islet and stem cell transplantation.