Introduction: Hydrogels have generated great interest to develop 3D artificial matrices that recapitulate features of the natural extracellular matrix (ECM) for applications in tissue regeneration or cell expansion[1],[2]. Covalent crosslinking is a common method to obtain stable hydrogels with precise control over crosslinking density. However, many of the crosslinking reagents used are toxic, precluding their use for cell encapsulation applications. Enzyme-mediated crosslinking reactions are finding increasing applications for developing hydrogels due to their mild conditions and enzyme specificity[3].
FXIIIa is a transglutaminase that catalyses the reaction between an ε-amine of lysine (K) onto a γ-carboxamide residue of glutamine (Q), resulting in the covalent bond formation of an ε-(γ-glutamyl)lysine isopeptide which is highly resistant to proteolytic degradation[4]. This crosslinking mechanism naturally occurs in vivo during the processes of wound healing. Hyaluronan (HA), a linear polysaccharide present in the ECM of many tissues, has been chemically modified to develop hydrogels[5]-[7]. In this work, HA was functionalized with two different transglutaminase peptide substrates to obtain HA hydrogels by enzyme-mediated crosslinking.
Materials and Methods: Peptides were synthesized manually using standard Fmoc based solid phase chemistry and purified, as described elsewhere[8].
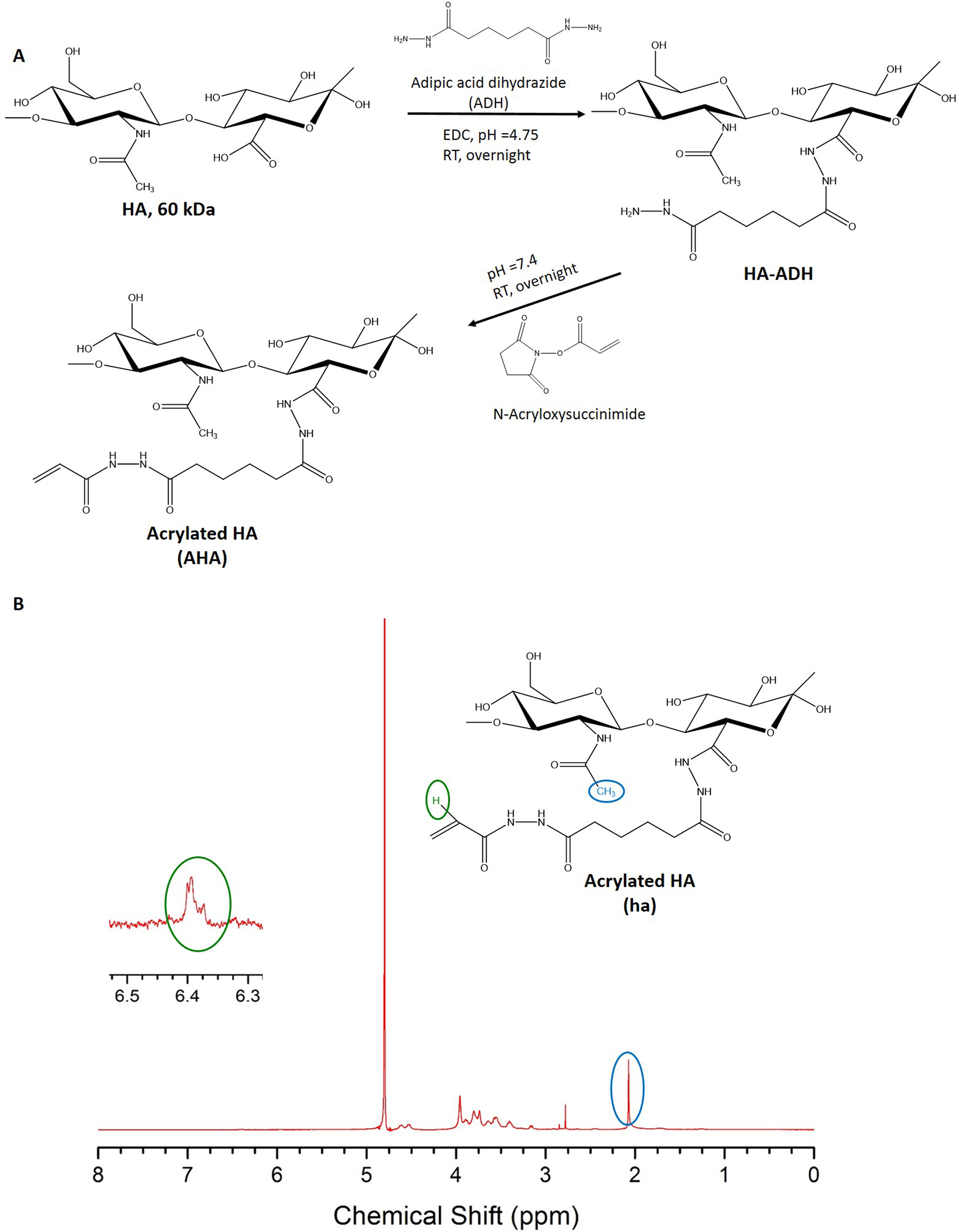
Hyaluronan (60 kDa) was functionalized with acrylate groups using a two-step synthesis (Fig 1-A, as described elsewhere[9].
Peptides were coupled to acrylated HA via Michael-type addition by reacting 1.2-fold molar excess of peptide over acrylate groups in 0.3 M triethanolamine (pH 8.0) overnight at 37°C.
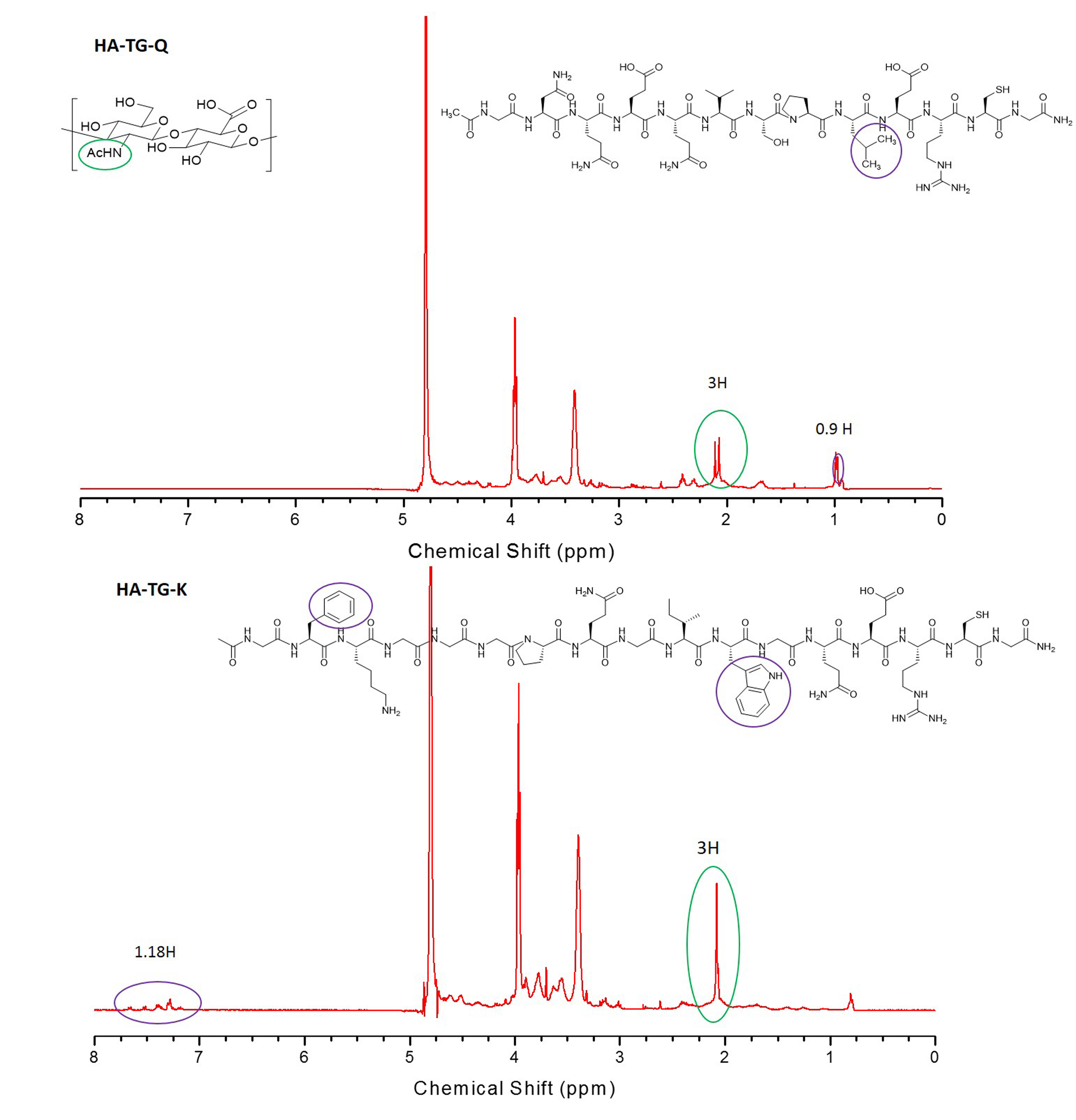
Results and Discussion: Peptides were designed as factor XIIIa substrates, containing either a lysine donor peptide CH3CONH-GFKGG-ERCG-CONH2 (TG-K, Mw = 950.08 g/mol) or a glutamine acceptor peptide - CH3CONH-GNQEQVSPL-ERCG-CONH2 (TG-Q, Mw = 1457.58 g/mol). In addition, a glutamine acceptor containing a cell adhesion ligand RGDS, which is recognized by several integrin receptors, was also synthesized, CH3CONH--GNQEQVSPLRGDSPG-CONH2 (TG-Q-RGDSPG, Mw = 1581.67 g/mol).
To render hydrogels susceptible to degradation by proteolytic enzymes, like natural fibrin, a lysine donor peptide was synthesized bearing a substrate for matrix metalloproteases (MMPs) Ac-GFKGGGPQGIWGQ-ERCG-CONH2 (TG-MMP-K, Mw = 1581.67 g/mol). Acrylated HA was obtained by a two-step reaction and the degree of acrylation was found to be 16%, as determined by NMR (Fig. 1-B). Transglutaminase peptide substrates were grafted onto acrylate groups of HA backbone via Michael-type addition as confirmed by NMR analysis (Fig. 2).
Conclusion: HA macromers functionalized with transglutaminase substrate peptides were successfully obtained. These HA-peptide macromers will be used as precursors to develop hydrogels that can be formed in situ (e.g. wound site), where FXIIIa is produced. The incorporation of integrin-specific adhesive ligands and cell-sensitive proteolytic domains in these hydrogels will aid in guiding and enhancing local tissue regeneration.
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007‐2013) under REA grant agreement n° 608765.
References:
[1] Griffith, L.G. and M.A. Swartz, Capturing complex 3D tissue physiology in vitro. Nature Reviews Molecular Cell Biology, 2006. 7(3): p. 211-224
[2] Lutolf, M.P. and J.A. Hubbell, Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology, 2005. 23(1): p. 47-55
[3] Moreira Teixeira, L.S., et al., Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials, 2012. 33(5): p. 1281-1290
[4] Lorand, L. and R.M. Graham, Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nature Reviews Molecular Cell Biology, 2003. 4(2): p. 140-156
[5] Burdick, J.A. and G.D. Prestwich, Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Materials, 2011. 23(12): p. H41-H56
[6] Dicker, K.T., et al., Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomaterialia, 2014. 10(4): p. 1558-1570
[7] Clark, R.A.F., K. Ghosh, and M.G. Tonnesen, Tissue engineering for cutaneous wounds. Journal of Investigative Dermatology, 2007. 127(5): p. 1018-1029
[8] Ferreira, D.S., et al., Hyaluronan and self-assembling peptides as building blocks to reconstruct the extracellular environment in skin tissue. Biomaterials Science, 2013. 1(9): p. 952-964
[9] Lam, J. and T. Segura, The modulation of MSC integrin expression by RGD presentation. Biomaterials, 2013. 34(16): p. 3938-3947