Introduction: BMP-2 is a highly potent growth factor that plays a crucial role in morphogenesis and tissue homeostasis [1],[2]. In vivo, BMP-2 exists both in solution and bound to the extracellular matrix (ECM), especially fibronectin (FN)[3]. While these two modes of presentation are known to influence cell behavior quite distinctly[4], their functional relevance has sparsely been investigated. Therefore, simple methods to create microenvironments presenting specific mechanical properties, ECM proteins and locally immobilized BMP-2 are important for elucidating fundamental features of developmental biology. In this study we used microcontact printing to obtain stable, cell-size micropatterns of BMP-2 alone or within fibronectin on soft biopolymeric films.
Materials and Methods: Film deposition was performed using poly(L-lysine) hydrobromide (PLL) and hyaluronic acid (HA) and cross-linking was achieved by using carbodiimide chemistry[5]. Polydimethylsiloxane (PDMS) stamps were made by casting Sylgard 184 liquid prepolymer over a silicon master fabricated by deep UV photolithography. Stamps were coated with BMP-2, FN alone or FN with BMP-2 (hereafter called FN/BMP-2), and placed in conformal contact with a (PLL/HA) film, before removal and cell deposition.
Results and Discussion: We generated micropatterns of BMP-2 on biopolymeric (PLL/HA) films, either alone or within FN (Fig. 1.A). We checked that the films were not damaged by confocal and atomic force microscopy. We quantified the amount of patterned BMP-2 and verified that BMP-2 was not released from the micropatterns. We observed a very selective adhesion of C2C12 myoblasts on the patterns of BMP-2 (Fig. 1.B). Cells presented a very specific cytoskeletal organization on our micropatterns, with very thick actin fibers along the sides of square patterns. Moreover, we observed that the presentation of BMP-2 by the pattern induced a significant recruitment of actin fibers around the nucleus.
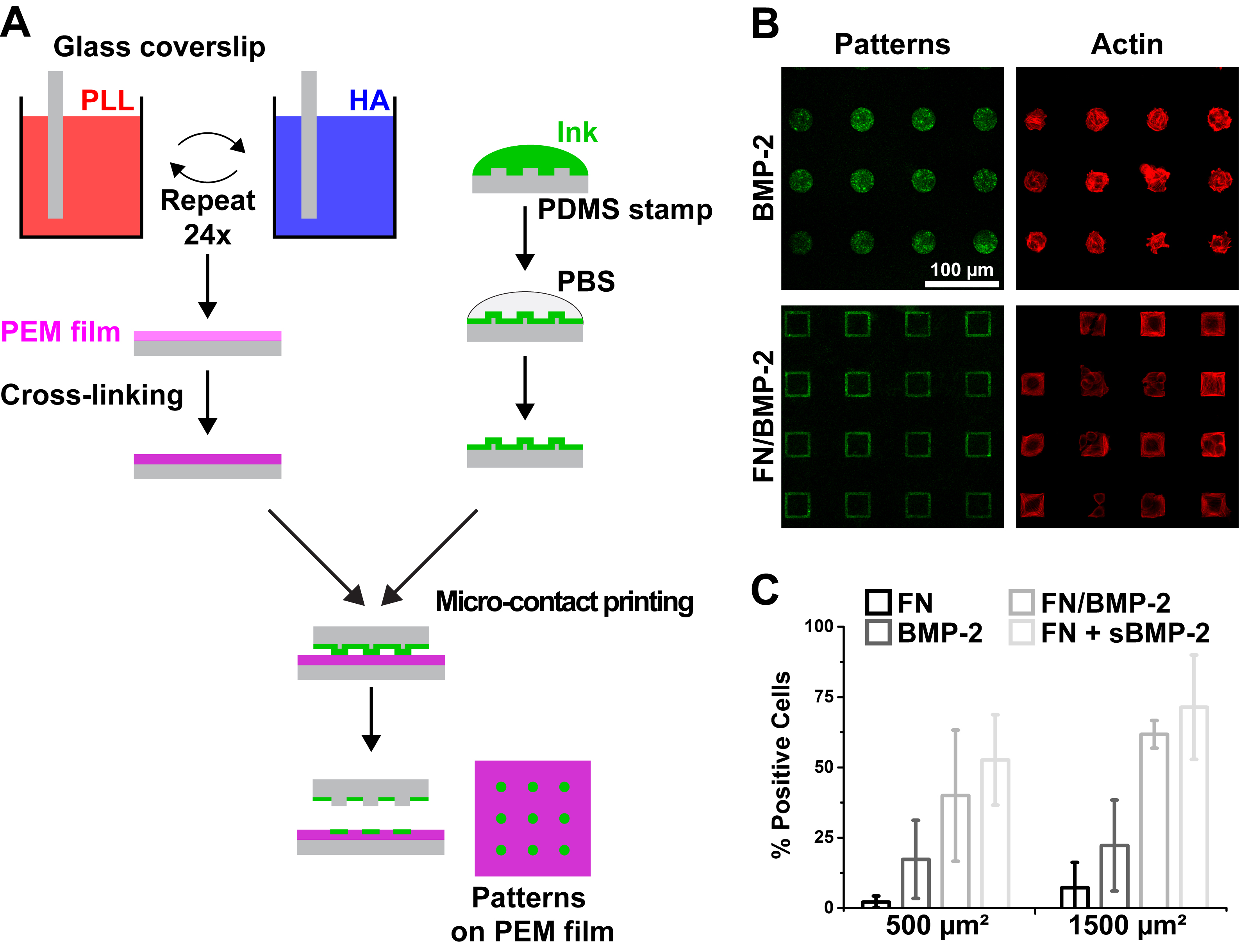
Fig. 1. Microcontact printing of BMP-2 on soft biopolymeric films. (A) Schematic of the film buildup combined with microcontact printing for generating micropatterns of FN or BMP-2 on soft (PLL/HA) films. (B) Representative images of C2C12 myoblasts (actin in red) on BMP-2 and FN/BMP-2 patterns (in green). (C) Percentage of positive cells with (i.e. p-SMAD1/5/8 is translocated to the nucleus) in function of the size and composition of the micropatterns.
We next investigated whether this effect was correlated with BMP-2-dependent regulation of target gene expression by analyzing the signaling of SMAD1/5/8 after 4 h of culture, a protein known to play a key role in the transduction pathway from BMP-2 receptors to the nucleus[6],[7]. Matrix-bound BMP-2 triggered the phosphorylation of SMAD1/5/8 and its translocation into the nucleus. We also observed that the normalized ratio of nuclear over cytoplasmic p-SMAD1/5/8 was significantly higher for myoblasts spread over large, 1500µm² patterns, compared to smaller, 500 µm² squares (Fig. 1.C). In order to confirm that this increased translocation of p-SMAD1/5/8 was indeed due to cell spreading, we compared the levels of p-SMAD1/5/8 in cells spread on solid versus hollow squares of FN/BMP-2. Although the amount of available BMP-2 in hollow squares was about a third of the amount in solid squares, the ratios of nuclear/cytoplasmic p-SMAD1/5/8 were similar for both conditions.
Conclusions: We demonstrated the possibility for the easy and versatile generation of cellular- and sub-cellular-sized patterns of unmodified BMP-2 on soft biopolymeric films, combined or not to FN. We unraveled the influence of matrix-bound BMP-2 on myoblast cytoskeletal organization, and the subsequent effect of cell shape on early trans-differentiation signaling.
The authors thank the members of the technical staff of the PTA cleanroom in Grenoble for their technical support; V.F. acknowledges support from the University Grenoble Alpes Ph.D. fellowship program; This work was supported by the European Commission, FP7 via an ERC Starting grant to C.P. (BIOMIM, GA 259370), by the Fondation pour la Recherche Médicale (C.A.R.) and by the Ligue Nationale contre le Cancer for Equipe labellisée Ligue 2014 (C.A.R.)
References:
[1] J. Kopf, P. Paarmann, C. Hiepen, D. Horbelt, P. Knaus, Biofactors 2014, 40, 171.
[2] B. L. M. Hogan, Genes Dev. 1996, 10, 1580.
[3] M. M. Martino, P. S. Briquez, E. Güç, F. Tortelli, W. W. Kilarski, S. Metzger, J. J. Rice, G. A. Kuhn, R. Müller, M. A. Swartz, J. A. Hubbell, Science 2014, 343, 885.
[4] J. Taipale, J. Keski-Oja, FASEB J. 1997, 11, 51.
[5] T. Crouzier, L. Fourel, T. Boudou, C. Albiges-Rizo, C. Picart, Adv. Mater. 2011, 23, H111.
[6] C. Sieber, J. Kopf, C. Hiepen, P. Knaus, Cytokine Growth Factor Rev. 2009, 20, 343.
[7] Y. K. Wang, X. Yu, D. M. Cohen, M. A. Wozniak, M. T. Yang, L. Gao, J. Eyckmans, C. S. Chen, Stem Cells Dev 2012, 21, 1176.