Introduction: Surgery in brachial plexus lesions is still a matter of debate and good results are generally difficult to achieve. The correct characterization of the topography and severity of the lesions and the timing for surgery are still open problems. In sharp-blade or gun-shot injuries there is the disruption of part of the structure; in this instance we suggest the use of artificial devices as tools to assist the reparative nerve regeneration and plexus reconstruction.
Peripheral nerve regeneration can be assisted by artificial devices named "nerve-guides" [1]. They are used in Humans to bridge single peripheral nerve gap-lesions. They are, basically, cylindrical conduits inside which a regenerating nerve stump may find protection and guidance; they perform at least as good as autografts in gaps not-longer-than 20 mm (bringing the advantage of avoiding donor site morbidity) [2]. Artificial nerve-guides have been applied in Brachial Plexus surgery in children [3] and adults [4].
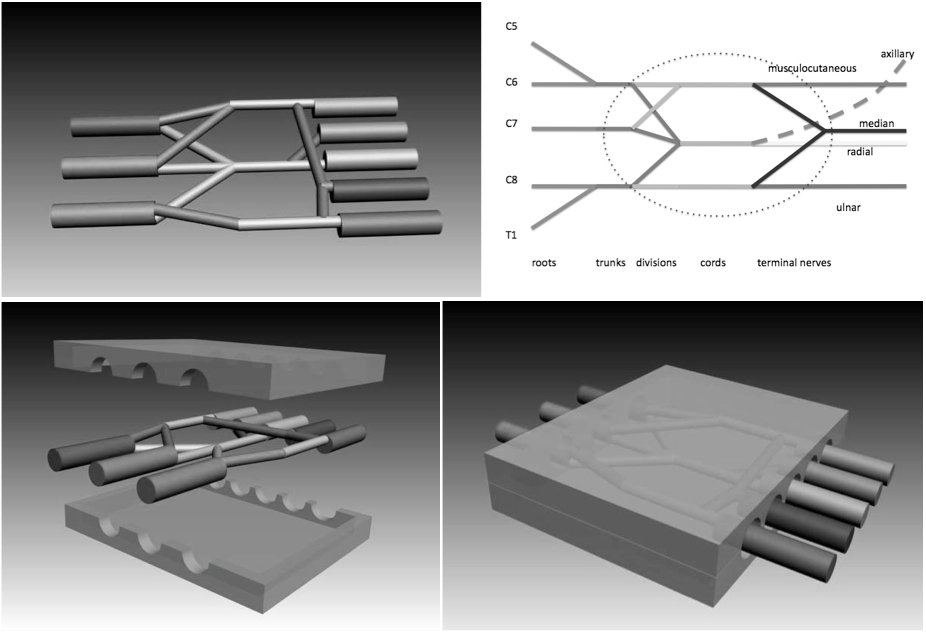
In 2006 we developed and tested in-vivo a new concept of box-shaped nerve-guide (called NeuroBox) which is double-halved and rigid, and does not require the use of any stitch to be sutured, allowing the use of cyanoacrylic glue instead [5],[6]. We devised a wider "NeuroBox" to be applied in disruptive lesions of the brachial plexus and we enterprised a search to choose a suitable animal model to test it (figure 1. top scheme: brachial plexus; bottom scheme: device protecting the brachial plexus space).
Materials and Methods: We replicated the surgical approaches to the brachial plexus described in the literature in: Wistar Rat; New Zealand White Rabbit; Sardinian Sheep (three animals each; bilateral). We decided not to replicate the approach in the Pig as we deemed adequate the analysis and discussion found in the literature. Measures were taken to minimize pain or discomfort to the animals and experiments were conducted in accordance with EU-Convention on the protection of animals revised directive 86/609/EEC. Surgical operation required the assistance of optical magnification and microsurgical instruments. Dimensions of the operative field were assessed by an intraoperative millimetric rule.
In evaluating the best animal model, we looked at: 1) cost of the animal and its mantainance; 2) standardization of the dimensions of the operative field; 3) possible translation to human surgical procedures; 4) difficulties of surgical technique in animal.
Results and Discussion: There is a relevant number of papers about experimental brachial plexus surgery in the Rats. However, we were unable to replicate any easy access to the different branchings of the plexus. Its dimensions were extremely small and were measured as 10+/-2 mm by side. This is a formidable difficulty in the perspective of producing and implanting an experimental device and in the aim of a possible translation of results in Humans.
The Rabbit was better suited, in respect to the Rat, for the implantation of an artificial device. However, the dimensions of the operative field were still pretty small, measuring 30+/-2 mm by side. The aim of a possible translation of results from the Rabbit to Humans seems limited by this difficulty.
The Sheep was good as far as dimensions of the operative field are considered, measuring 60+/-10 mm by side. The surgical access was easy because of the dimensions and the lack of the clavicole (which is a peculiarity to this animal specie). The translation of results form the Sheep to the Human is feasible because of the dimensions of the operative field and of the device eventually used.
In the literature, the Pig presents with a wider brachial plexus in comparison with the sheep, however its standardization seems difficult in serial experiments since its dimensions continue to increase with growth. Furthermore, its costs are the highest among all the models analyzed so far.
Conclusions: Dimensions, reproducibility, easy surgical access, affordable costs are among the advantages of the sheep animal model in the study of artificial nerve regeneration in brachial plexus lesions. Based on these findings, we started the experimental implantation of NeuroBox devices in the Sardinain Sheep model.
References:
[1] Merolli A. Nerve-conduits or nerve-guides? When terminology matters. Injury 2013; 44: 878-879
[2] Schlosshauer B, et Al. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 2006; 59: 740-747
[3] Ashley WW, et Al. Collagen nerve guides for surgical repair of brachial plexus birth injury. J Neurosurg 2006; 105: 452–456
[4] Wolfe SW, et Al. Use of bioabsorbable nerve conduits as an adjunct to brachial plexus neurorrhaphy. J Hand Surg Am 2012; 37(10): 1980-1985
[5] Merolli A, et Al. In-vivo regeneration of rat sciatic nerve in a double-halved stitch-less guide: a pilot study. Microsurgery 2009; 29: 310-318
[6] Merolli A, et Al. In vivo study of ethyl-2- cyanoacrylate applied in direct contact with nerves regenerating in a novel nerve- guide. J Mater Sci Mater Med 2010; 21: 1979-1987