Introduction: Over 1.7 million people sustain a traumatic brain injury (TBI) annually, costing $76.5 billion in the U.S. alone[1]. TBI is initiated by a mechanical injury and leads to a biochemical injury that is largely responsible for long-term deficits. Stem cell transplants have shown some success mitigating the biochemical injury in preclinical studies, but suffer low retention and engraftment rates(2-4%)[2]. Therefore, transplants need a more permissive microenvironment to improve their retention and engraftment after TBI. The neural niche, which is comprised largely of hyaluronic acid (HA) and is highly vascularized, may be replicated by engineering a transplant environment comprised of HA and basement membrane protein laminin. Both HA and laminin have been shown to work in concert with the chemokine stromal cell-derived factor-1alpha (SDF-1a), which is upregulated in the injury penumbra. Specifically, laminin-SDF-1a crosstalk increases NPSC migration[3] and HA interaction upregulates the SDF-1a receptor, CXCR4, in NPSCs[4]. Therefore motivating the development of a transplant scaffold that 1. enhances NPSC sensitivity to SDF-1a and 2. increases NPSC migration into the surrounding host tissue.
Methods: HA-laminin (HA-Lm) gels were crosslinked with poly(ethylene glycol) divinyl sulfone (PEGDVS), where laminin was covalently immobilized to PEGDVS. NPSC chemotactic migration was assessed by a transwell assay, where NPSCs seeded on top of the gels were exposed to an SDF-1a gradient for 24 and 48 hrs and migrated cells visualized with DAPI. NPSC chemotactic migration in vivo was assessed in response to exogenous SDF-1a. All animal work was approved by the ASU Institutional Animal Care and Use Committee. C57/BL6 mice received two injections into the intact brain: 1. NPSCs either bolus or within the HA-Lm gel and 2. exogenous SDF-1a that was 0.5 mm lateral of the NPSC injection. NPSCs were labeled with QTracker 655 to allow for analysis of cell retention and migration out of the NPSC needletrack at 1 and 3 days after transplantation.
Results and Discussion: NPSC in vitro chemotactic migration significantly increased after 48 hrs of culture on HA-Lm gel when exposed to an SDF-1a gradient compared to uniform or no SDF-1a (data not shown). As such, the HA-Lm gel provides the appropriate infrastructure to promote NPSC migration in response to the SDF-1a signaling for which it primes NPSCs, serving as a dual-purpose scaffold for transplantation. HA-Lm gel significantly increased NPSC transplant retention within the brain at 1 and 3 days compared to bolus NPSC transplants (Figure 1). Moreover, in vivo NPSC migration in response to exogenous SDF-1a significantly increased when transplanted in the HA-Lm gel compared to bolus at both 1 and 3 days (Figure 2), providing robust motivation for its use as a transplantation scaffold following TBI.
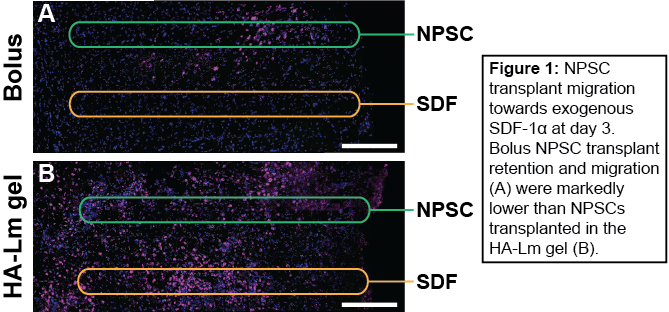
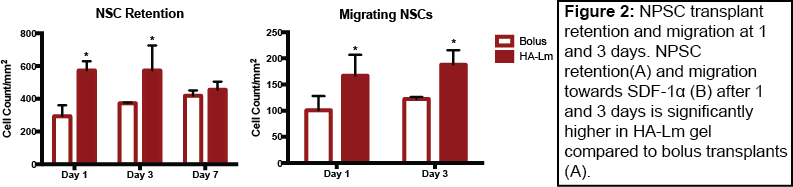
Conclusion: Our transplantation platform has been designed to provide transplants with a permissive and niche-mimetic transplantation environment while simultaneously taking advantage of local endogenous repair signaling and its effect on exogenous transplants. The results presented here pave the way for development of an improved cell therapy for traumatic brain injury, in which transplants are afforded higher rates of retention and engraftment within the surrounding host tissue.
Emma Goddery; Sanjay Srinivasan; NIH New Innovator (NIH1DP2HD084067)
References:
[1] V.G. Coronado, L.C. McGuire, K. Sarmiento, J. Bell, M.R. Lionbarger, C.D. Jones, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009 J. Saf. Res., 43 (2012), pp. 299–307
[2] M.T. Harting, J.E. Baumgartner, L.L. Worth, L. Ewing-Cobbs, A.P. Gee, M.-C. Day, et al. Cell therapies for traumatic brain injury Neurosurg. Focus, 24 (2008), p. E18
[3] C.P. Addington, C.M. Pauken, M.R. Caplan, S.E. Stabenfeldt The role of SDF-1α-ECM crosstalk in determining neural stem cell fate Biomaterials, 35 (2014), pp. 3263–3272
[4] C.P. Addington, J.M. Heffernan, C.S. Millar-Haskell, E.W. Tucker, R.W. Sirianni, S.E. Stabenfeldt. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials, 73 (2015), pp.11-19