Introduction: Women and children suffer harsh consequences of gonadal dysfunction that arise from cancer treatments therapies and developmental disorders[1]. Current treatment options are limited and only restore short-term gonadal function. Great advances in ovarian follicle culture and transplant, including live birth in mice, have been made with biomaterials (hydrogel bead encapsulation)[2].These strategies, however, do not permit advanced design to optimize transplant function. Therefore, we are investigating an advanced fabrication method, 3D printing (3DP), towards developing an artificial ovary for long-term fertility and hormone options.
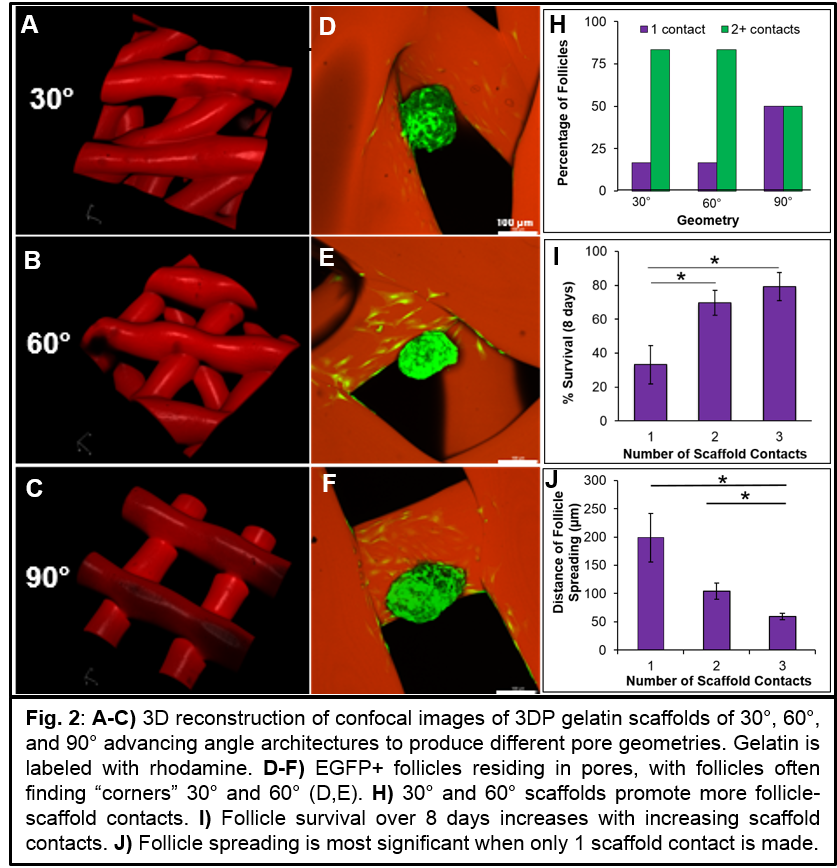
Methods and Materials: Printing and material parameters were optimized using a 3D-Bioplotter. Gelatin scaffolds of various pore geometries (30°,60°,90° advancing angle Fig.2A-C) were EDC/NHS cross-linked, and architecture was assessed by light and fluorescence microscopy. Secondary murine follicles were mechanically isolated from excised ovaries and seeded into scaffolds. Follicle viability was assessed by the appearances of a healthy oocyte and intact basement membrane as observed by light microscopy. Number of contacts with scaffold and length of follicle spreading was quantified in 3D reconstructions of confocal stacks. Estradiol content of media was assessed by ELISA. After eight days, follicles were exposed to hCG to mimic the murine estrous cycle and induce ovulation.
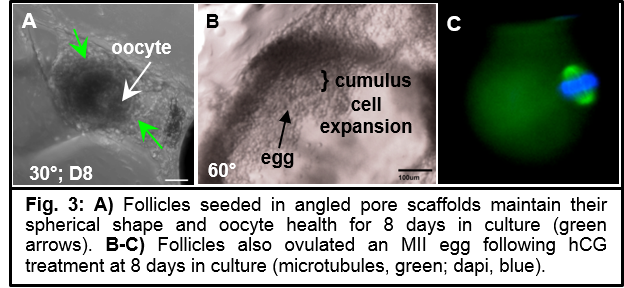
Results and Discussion: Utilizing thermogelation of gelatin, we print well-defined and multi-layered scaffolds (Fig.1). To probe the utility of 3DP scaffolds for creating an artificial ovary, we cultured follicles in scaffolds. Follicle survival was best promoted in angled, trapezoidal pores (30°, 60°) as opposed to square pores (90°) (Fig.2I). We determined that the mechanism of increased survival in angled pores was that these scaffold architectures increased the likelihood of the follicles to make multiple scaffold contacts (Fig.2H), thus creating a “3D experience” to maintain follicle sphericity (Fig.2D-F,J). Follicles were also functional, continuously producing estradiol over the culture period and when stimulated, ovulated a mature egg within the scaffold (Fig.3). Most importantly, this did not require any biomaterial degradation intervention to release the egg as is needed when using hydrogel beads.
Conclusions: This work is the first to demonstrate 3DP scaffolds as an artificial environment for supporting follicle health and function, and we are the first to report in vitro ovulation from a biomaterial. Significantly, 3DP provides easy scalability for human-scale grafts, material and scaffold architecture customization, and open and interconnected porosity to permit vascularization and egg release into uterine tubes during ovulation for eventual fertilization. These findings encourage further evaluation of 3DP scaffolds towards developing a functional and clinically translatable artificial ovary.

References:
[1] Jeruss J, Woodruff, T. N Engl J Med. 2009:360(9):902-11
[2] Xu M et al. Tissue Engineering. 2006;12(10):2739–2746.