Introduction: Recently, in-situ recruiting bone marrow mesenchymal stem cells (BMSCs) have become a promising approach for promoting bone regeneration, especially in the non-union bony fracture sites. However, maintaining a considerable number of cells with appropriate phenotype remains challenging. As CD271 antibody have high specificity toward human BMSCs (hBMSCs), in this study we attempted to prepare chitosan microspheres functionalized with CD271 antibody for efficient isolation and capture of hBMSCs, which can be used as the building blocks to facilitate bone defect repair.
Materials and Methods: The chitosan microspheres functionalized with CD271 antibody were prepared through stepwise modification with biotin, streptavidin, and biotinylated CD271 antibody. These microspheres were then divided into control group (no antibody), low concentration group, and high concentration group according to the concentration of CD271 antibody. The amount of CD271 antibody on the microspheres was assessed by conjugation with a Cy3-labeled secondary antibody. The ability of hBMSCs capture and expansion by the microspheres from a homotypic cell population was examined. Cell selection/isolation from a heterotypic cell suspension which was mixed with hBMSCs and mice osteosarcoma cells at a 1:1 ratio was also measured. These two types of cells were pre-stained with different fluorescent labels for further observation.
Results and Discussion: The mean diameter of chitosan microspheres was about 300 μm. The microspheres of high concentration group showed higher fluorescence intensity, indicating that more CD271 antibody was conjugated on them (Fig. 1A). The chitosan microspheres functionalized with CD271 captured more hBMSCs than those in the control group (without CD271), and the microspheres in the high concentration group captured even more hBMSCs than those in low concentration group (Fig. 1B). In a heterotypic cell suspension mixed with hBMSCs and mice osteosarcoma cells, the microspheres modified with CD271 antibody could isolate hBMSCs in a antibody concentration-dependent manner (Fig. 2A). In addition, the cells could adhere and expand on the surface of microspheres functionalized with CD271 antibody, but not on non-modified microspheres. During culture, the number of cells kept increasing on the microspheres of the high concentration group (Fig. 2B).
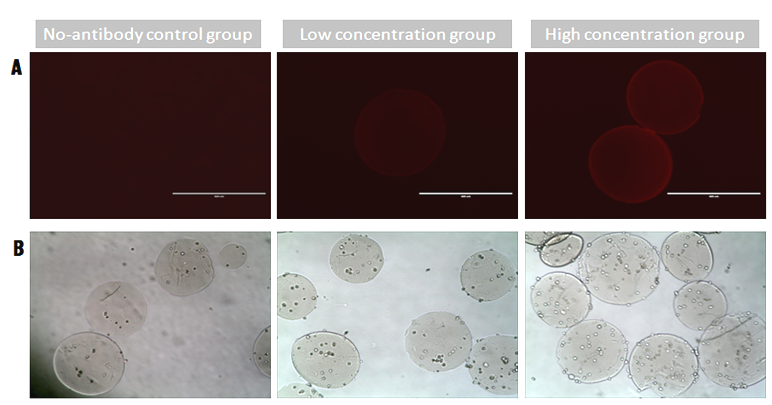
Figure 1. (A) Functionalized microspheres incubated with a secondary antibody Cy3. (B) hBMSCs captured by the microspheres after 3h culture.
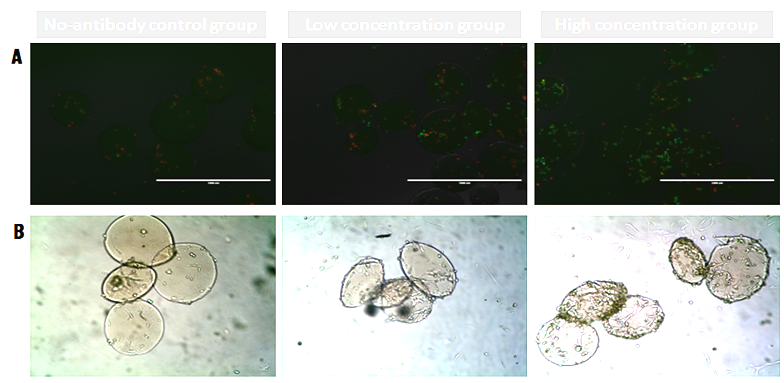
Figure 2. (A) Cell isolation by functionalized microspheres (hBMSCs, green; mice osteosarcoma cells, red). (B) hBMSCs expansion on the microspheres after 7 days of culture.
Conclusion: The chitosan microspheres functionalized with CD271 antibody were able to isolate and capture hBMSCs, and supported the proliferation of cells as well. Such antibody-modified microspheres showed great potential as scaffolds to recruit BMSCs in-situ and as a result, to promote their proliferation and differentiation to promote new bone formation and bone defect repair.
National Natural Science Foundation of China (81171479, 51303120, 81471790); Jiangsu Provincial Special Program of Medical Science (BL2012004); Natural Science Foundation of Jiangsu Province (BK20130335)