Introduction: Chemotherapy is one of the most important arsenals against cancer, but always results in undesired purgatorial side-effects such as hair loss, bone marrow suppression and organ damage. Tumor-activatable prodrugs that are selectively activated in tumors were developed to reduce off-target toxicity and improve the therapeutic index. Real-time monitoring when, where, and how the prodrugs are activated in vivo is critical for their development, but it remains as a great challenge since we lack capable theranostic prodrugs to track their behavior in vivo. The reported fluorescent theranostic prodrugs were plagued by their mono-channel fluorescence and short wavelength, which were insufficient for simultaneously tracking their biodistribution and activation in vivo[1],[2].
To solve the problem, we developed a novel theranostic prodrug (Cy-S-CPT) from cyanine dye and anticancer drug CPT, which were covalently connected with a disulfide bond linkage. The cleavage of the disulfide bond by GSH and the successive cyclization reaction produced the activated anti-cancer drug CPT and induced remarkable fluorescent shift from 825 to 650 nm, making a breakthrough with switchable dual-channel near-infrared (NIR) fluorescence to real-timely scrutinize the activation process of prodrugs with disulfide bond linker in vivo (Figure. 1).
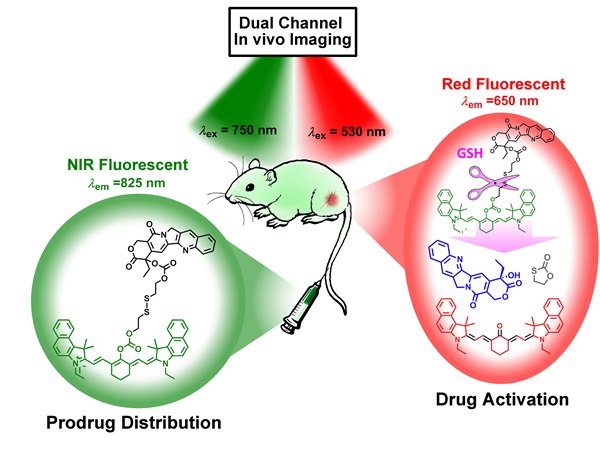
Figure. 1 Design and activation mechanism of dual-channel fluorescent theranostic prodrug
Results and Discussion: The prodrug release process was tested at different conditions by using spectrometer, flow cytometer and confocal laser scanning microscopy, which verified the activation sensitivity and thiol-specificity in vitro. The in vivo fluorescent imaging of mice after the intravenous injection of the prodrug loaded in PEG-PLA nanoparticles was performed with different fluorescence channels (λex = 750 nm and λem = 825 nm vs λex = 530 nm, λem = 650 nm ) (Figure. 2B). Clearly, the NIR fluorescence of intact prodrug (represented in green) was quickly distributed in the whole body of mice soon after the injection, and then slowly faded out, demonstrating a quick distribution and gradual activation of Cy-S-CPT. Simultaneously, the red fluorescence of cyanine dye (represented in red) became stronger as time increased, and reached its peak at 4 h post injection, which indicated the highest prodrug activation level occurred at this time point. Intriguingly, even though red fluorescence dispersed broadly in the mice, indicating a comprehensive activation occurred in all organs, the tumor bestowed the strongest red fluorescence at 24 h post injection, which revealed a preferential prodrug accumulation and activation in tumor. Additionally, the in vivo antitumor studies performed on xenografted BCap-37 tumor model indicated that Cy-S-CPT loaded nanoparticles displayed significantly improved therapeutic efficacy than the clinical used drug CPT-11(Figure. 2B).
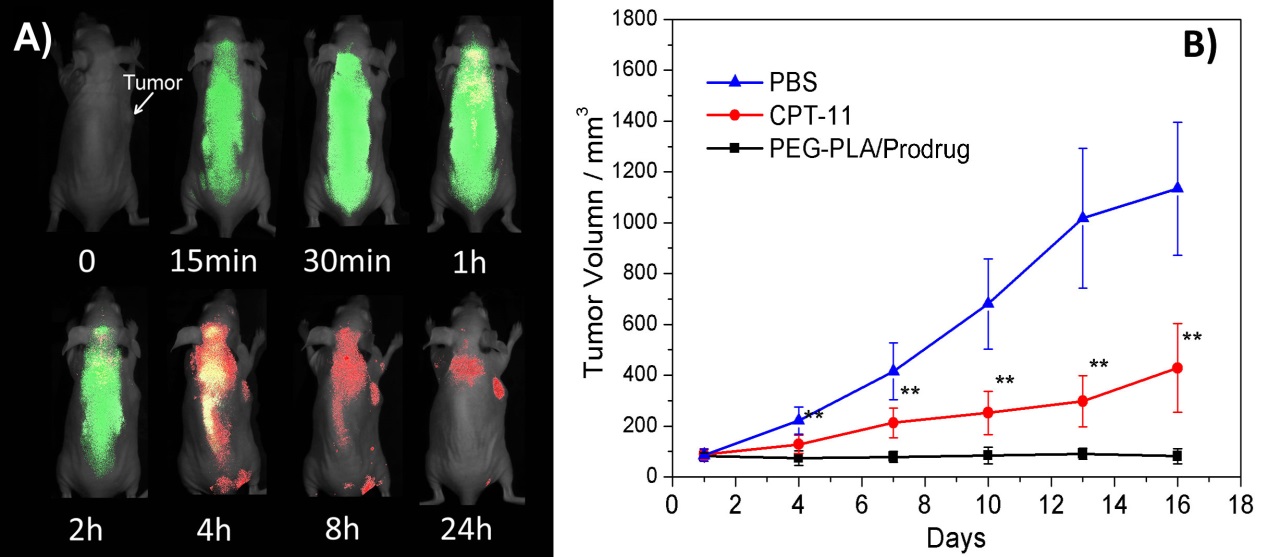
Figure. 2 In vivo imaging (A) and antitumor activities (B) of the theranostic prodrug on BCap-37 tumor xenografted nude mice. Note: green signal represents the fluorescence from the intact prodrugs (λex = 750 nm, λem = 825 nm), indicating prodrug biodistribution; while red signal represents the fluorescence from CyA-K (λex = 530 nm, λem = 650 nm), indicating drug activation. Yellow represents the mix.
Conclusion: A theranostic prodrug with a disulfide bond linkage possessing switchable NIR fluorescent from 825 to 650 nm was prepared and used to investigate GSH-responsive drug activation in vivo. The dual-channel fluorescent imaging indicated that although the prodrug with disulfide could be activated comprehensively in all the organs and tissues with a relatively high speed, tumor demonstrated the strongest prodrug activation at 24 h post injection. Moreover, Cy-S-CPT exhibited significant therapeutic efficacy which overwhelmed clinical used drug CPT-11. The theranostic prodrug with switchable dual-channel near-infrared (NIR) fluorescence provided a new strategy to real-time monitor the distribution and activation of prodrugs in vivo.
National Basic Research Program (2014CB931900and 2013CB733700); NSFC for Creative Research Groups (21421004) and Distinguished Young Scholars (21325625), NSFC/China
References:
[1] Yang, Z.; Lee, J. H.; Jeon, H. M.; Han, J. H.; Park, N.; He, Y.; Lee, H.; Hong, K. S.; Kang, C.; Kim, J. S. Journal of the American Chemical Society 2013, 135, 11657
[2] Wu, X. M.; Sun, X. R.; Guo, Z. Q.; Tang, J. B.; Shen, Y. Q.; James, T. D.; Tian, H.; Zhu, W. H. Journal of the American Chemical Society 2014, 136, 3579