Artificial extracellular matrix - a promising approach to enhance new bone formation in critical size bone defects
-
1
University Hospital "Carl Gustav Carus" at TU Dresden, UniversityCenter of Orthopedics and Trauma Surgery, Germany
-
2
TU Dresden, Institute of Materials Science, Max Bergmann Center of Biomaterials, Germany
-
3
Innovent e.V. Jena, Department Biomaterials, Germany
Bone regeneration in critical size bone defects still represents an important but unsolved clinical problem. Still, autologous bone transplantation is the medical gold standard which is associated with donor site morbidity and limited resources. Biodegradable scaffolds are a promising alternative for bone regeneration as they provide porous matrices that can support cell adhesion, proliferation, and differentiation. The addition of specific coatings can improve the osteoconductive and osteoinductive properties of these scaffolds. Previous studies have shown the successful use of components of the organic extracellular matrix (ECM) like collagen type I (Coll) and glycosaminoglycans (GAGs) in order to create a favorable microenvironment for bone forming cells and their progenitors. An artificial ECM (aECM) consisting of Coll and chondroitin sulfate (CS) or sulfated hyaluronan (sHA) improved osteogenic differentiation of mesenchymal stromal cells in vitro[1],[2]. The aim of the present study was to investigate the effect of these a ECM on new bone formation in a critical size bone defect in vivo.
A 5 mm defect was created in the femur of 84 adult male Wistar rats and stabilized with an internal fixator and randomly divided into 7 groups of 12 animals each. Embroidered PCL (polycaprolactone-co-lactide) scaffolds were coated with Coll, Coll/CS, Coll/hyaluronan (Coll/HA), Coll/oversulfated CS (Coll/sCS3, degree of sulfation (DS) ≈ 3), and Coll/sHA3 (DS ≈ 3). Uncoated PCL scaffolds served as negative controls, and collagen sponges with bone chips to mimic autologous bone graft served as positive controls. Six rats of each group were sacrificed after 2 and 12 weeks. New bone formation and the quality of the newly formed bone were evaluated by radiographs, micro computed tomography (µCT), and histological staining.
Histological staining showed no signs of inflammation indicating a good biocompatibility of the coatings. Radiographs and µCT measurements showed nearly no formation of calcified tissue within the scaffolds at 2 weeks after implantation. HE staining at 2 weeks showed incipient ossification from the bone ends with small clusters of newly formed woven bone for Coll/CS- and Coll/sHA-coated scaffolds indicating an earlier bone remodeling compared to the controls.
The new bone volume after 12 weeks was 77%±20% for the positive control, 51%±19% for the uncoated PCL, 46%±30% for Coll, 45%±27% for Coll/CS, 47%±30% for Coll/HA, 57%±24% for Coll/sCS3, and 73%±38% for Coll/sHA3. Thus, scaffolds coated with Coll/sHA3 displayed the same amount of new bone formation as the positive control.
In this study we could demonstrate that the PCL scaffolds coated with an artificial ECM consisting of Coll and highly sulfated HA have the same effect on new bone formation as autologous bone grafting in a critical size bone defect in rats. In a clinical setting such scaffolds could potentially spare the patients an additional surgery for bone harvesting.
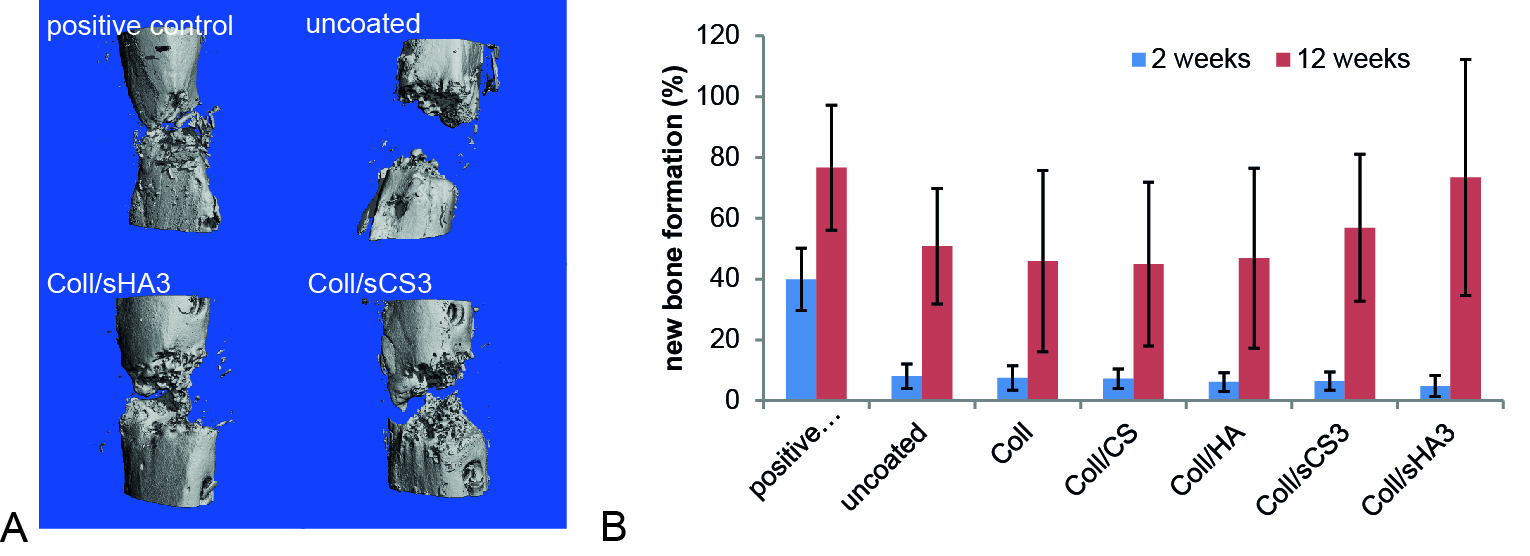
Fig. 1: A) µCT images of selected coatings to illustrate the distribution of the new bone within the defect area at 12 weeks after implantation. B) Quantification of the new bone formation after 2 and 12 weeks of implantation. The contralateral section of the femur was used as reference and set to 100%. Data are represented as mean±SD.
This study was supported by grants from Deutsche Forschungsgemeinschaft Transregio 67 (subprojects A3, B5, and Z3). The authors thank Heike Zimmermann, Aline Katzschner, Suzanne Manthey, and Annett Wenke for technical assistance.
References:
[1] Hempel U. et al. (2014) Biomed Res Int. 2014: 938368
[2] Hess R. et al. (2012) Biomaterials 33: 8975-85.
Keywords:
Bone Regeneration,
Extracellular Matrix,
Regenerative Medicine
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Regeneration inducing biomaterials
Citation:
Förster
Y,
Hintze
V,
Möller
S,
Schnabelrauch
M,
Scharnweber
D and
Rammelt
S
(2016). Artificial extracellular matrix - a promising approach to enhance new bone formation in critical size bone defects.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02353
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.