Introduction: Inflammatory bowel disease (IBD), which has two main forms: Crohn’s disease and ulcerative colitis (UC), is a chronic inflammatory disorder affecting the gastrointestinal tract[1]. To date there is no cure for IBD. The treatment goal is to rapidly control the flares and maintain a long-lasting remission phase that requires life-long medication. Current therapies for IBD do not confine drugs to the site of action, which contributes to systemic drug absorption leading to severe side effects and low patients adherence[2]. Delivering drugs to patients in a safe, effective, and compliant manner poses considerable challenges in IBD treatment. Conventional colon-targeting systems deliver and release drugs to the colon, but not necessarily target sites of inflammation. We developed an inflammation-targeting hydrogel that selectively targets inflamed colon to improve treatment efficacy, reduce side effects and potentially promote medication adherence[3].
Materials and Methods: The hydrogel microfibers were made from ascorbyl palmitate, one of the “Generally Recognized as Safe” (GRAS) materials listed by the Food and Drug Administration (FDA). Dexamethasone (Dex) was used as a model drug, and the release of Dex from the hydrogel in response to enzymes upregulated under inflammation was quantified in vitro. The preferential adhesion of hydrogel microfibers to inflamed colon was demonstrated ex vivo and in vivo using a fluorescent dye encapsulated into the hydrogel for imaging purpose. Two murine colitis models, T-bet−/−Rag2−/− ulcerative colitis (TRUC)[4],[5] and dextran sodium sulfate (DSS) induced colitis[6], were used for animal studies. The therapeutic efficacy of Dex-loaded hydrogel administered to colitic mice via enema was compared with Dex alone, hydrogel alone or untreated mice. Systemic Dex exposure in serum after a single enema of Dex-loaded hydrogel or Dex alone to colitic mice was quantified at different time points. Colon tissue biopsies from UC patients were collected and analyzed for hydrogel selective adhesion to inflamed mucosa compared with normal mucosa.
Results and Discussion: The self-assembled hydrogel demonstrated enzymatically triggered release of Dex, and showed preferential adhesion to inflamed epithelial surfaces in vitro and in mouse colitis models in vivo. Dex-loaded hydrogel significantly inhibited colon inflammation in the TRUC model in comparison with hydrogel alone, Dex alone and untreated controls (Fig. 1). Dex quantification in the serum indicated an 8-15-fold lower Dex peak serum concentrations and 4-7-fold less systemic drug exposure after a single enema of Dex-loaded hydrogel compared with Dex alone. Ex vivo analysis of biopsy specimens from UC patients demonstrated that the hydrogel preferentially adhered to inflamed lesions compared with histologically normal sites (Fig. 2), consistent with our mouse data.
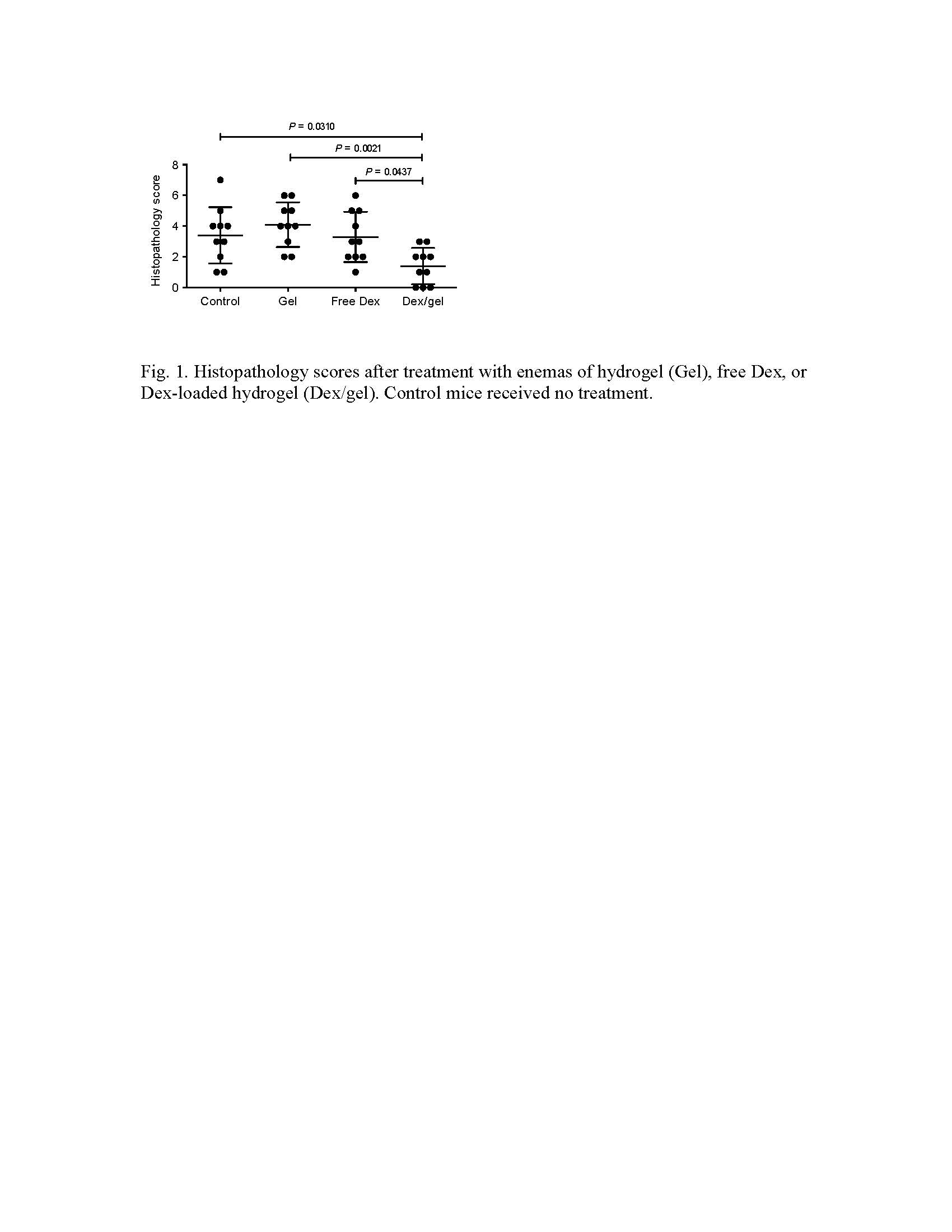
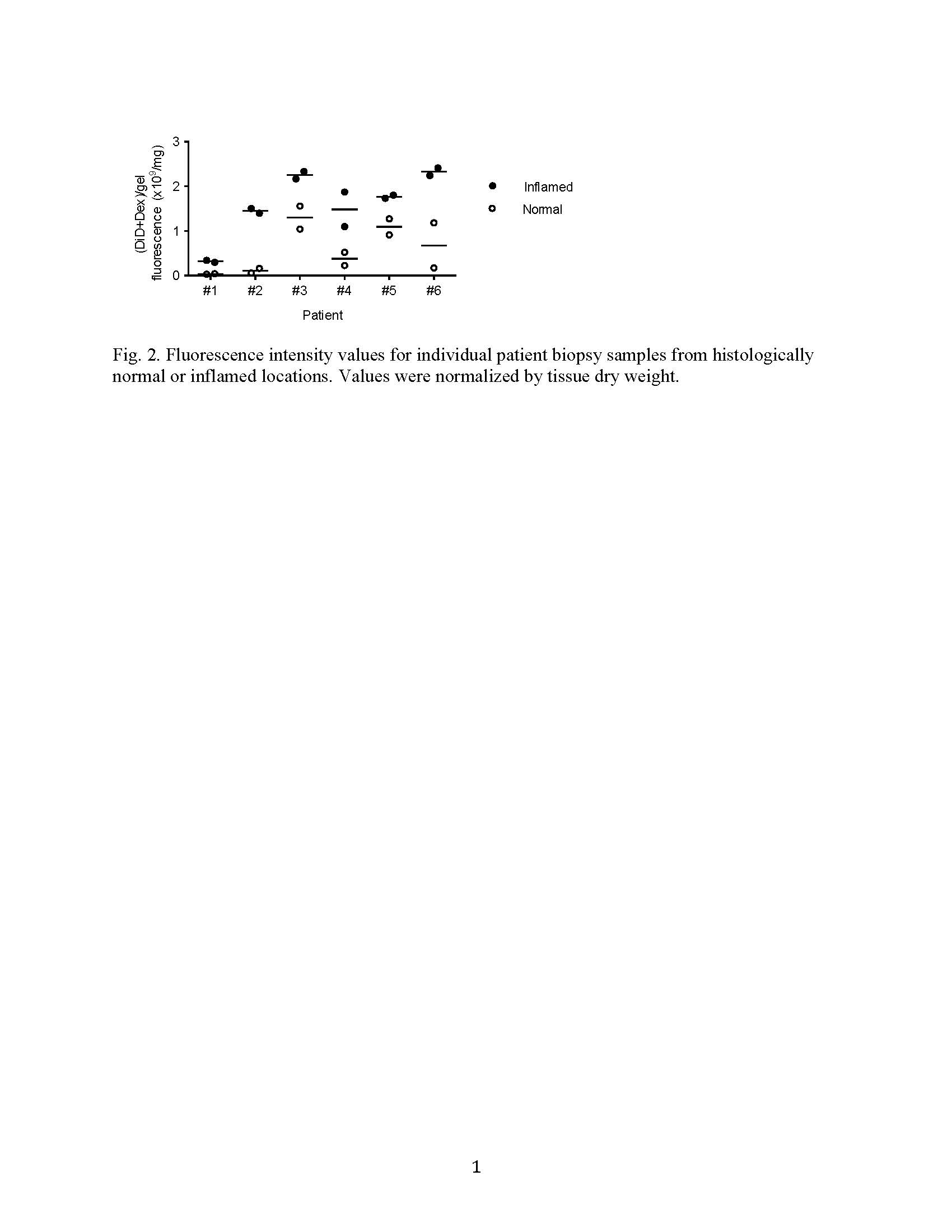
Conclusion: We have developed a strategy for targeted drug delivery to inflamed colon using a GRAS hydrogel via rectal administration. The selective adhesion of GRAS hydrogel to the inflamed mucosa was demonstrated in two mouse models and with biopsies from UC patients. Dex-loaded hydrogel was able to enhance the treatment efficacy and significantly reduce the systemic Dex exposure. This system has the potential to prolong local drug availability, reduce dosing frequency and promote patient adherence. Our targeted drug delivery platform represents a promising approach to improve enema-based therapies in patients with colonic IBD.
Natural Sciences and Engineering Research Council of Canada; Ramalingaswami Re-entry Fellowship from the Department of Biotechnology, India; Harvard Institute of Translational Immunology/Helmsley Trust Pilot Grants in Crohn’s Disease; National Institutes of Health; Max Planck Research Award from the Alexander von Humboldt Foundation
References:
[1] Kaser A, Zeissig S and Blumberg R S 2010 Inflammatory bowel disease Annu Rev Immunol 28 573-621
[2] Kane S V 2006 Systematic review: adherence issues in the treatment of ulcerative colitis Aliment Pharmacol Ther 23 577-85
[3] Zhang S, Ermann J, Succi M D, Zhou A, Hamilton M J, Cao B, Korzenik J R, Glickman J N, Vemula P K, Glimcher L H et al. 2015 An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease Sci Transl Med 7 300ra128
[4] Ermann J, Staton T, Glickman J N, de Waal Malefyt R and Glimcher L H 2014 Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet-/-.Rag2-/- (TRUC) mice Proc Natl Acad Sci U S A 111 E2559-66
[5] Garrett W S, Lord G M, Punit S, Lugo-Villarino G, Mazmanian S K, Ito S, Glickman J N and Glimcher L H 2007 Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system Cell 131 33-45
[6] Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman S V and Merlin D 2009 Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis PLoS One 4 e6073