Introduction: Inorganic polyphosphate (polyP) is a polymer that is widespread in biology and has many functions. In particular, it is secreted by activated platelets and accelerates blood clotting. As understanding of polyP’s role expands, so does the desire to incorporate polyP into novel biomaterials as hemostatic agents. We previously reported that the terminal phosphates of polyP can form phosphoramidate bonds with primary amines via 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC)-mediated reactions, albeit under conditions that resulted in some polyP hydrolysis. We now report improvements to avoid polyP hydrolysis, improve yield, and increase the range of possible conjugates for covalently end-labeling polyP. This will facilitate development of new materials to which polyP is covalently attached, as well as analyses of polyP’s biological effects.
Methods: PolyP (100mM monophosphate) was incubated 1 h at 37°C with EDAC (150mM) in pH 8.0 MOPS buffer and a bridging conjugate containing a primary amine together with another functional group (reducible disulfide, alkyne or additional primary amine; 8mM). Once attached to polyP, the bridging conjugates were reacted with a second reagent (8mM) via a complementary reactive group (maleimide, azide, or N-hydroxysuccinimide ester) attached to a desired payload (biotin, a fluorophore or a peptide epitope tag). Biotinylation allowed rapid evaluation of labeling efficiency using a solid-phase thrombin-binding assay.
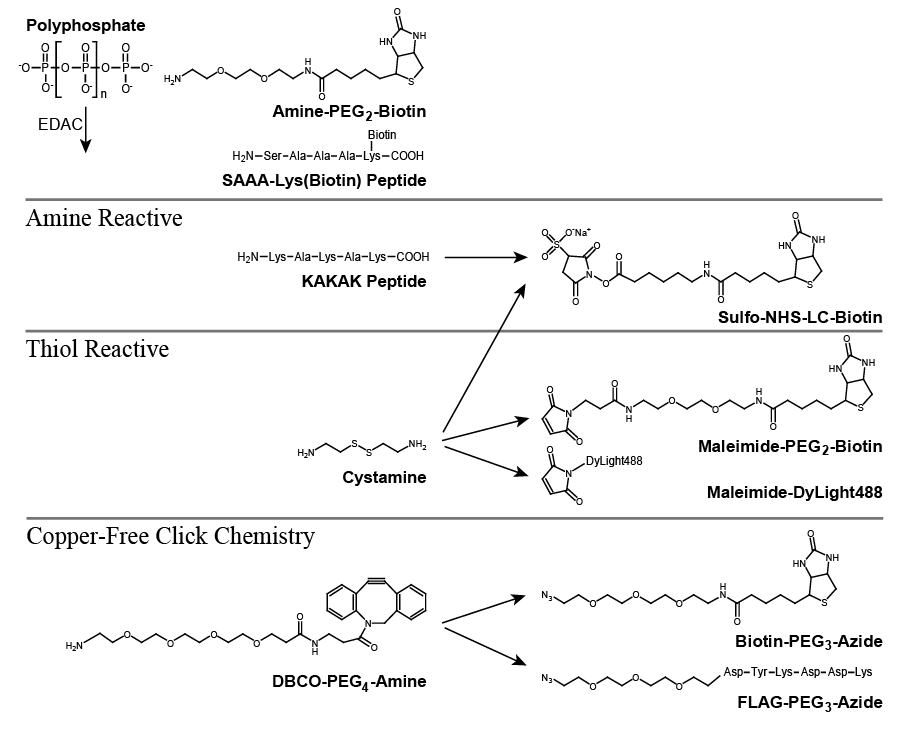
Results: Optimized reaction conditions allowed rapid, efficient, covalent linkage of amine-PEG2-biotin to polyP without decreasing polymer length. Synthetic peptides make effective bridges, as they are easily synthesized and customized. Accordingly, polyP was conjugated to two peptides: SAA-Lys(biotin) via its free N-terminus; or KAKAK via random coupling, after which remaining free amines were coupled to sulfo-NHS-LC-biotin. Cystamine-labeled polyP was reduced and immediately reacted with maleimide-PEG2-biotin or maleimide-DyLite-488 fluorescent dye. Biotin-PEG3-azide or azide-PEG3-FLAG epitope tag were attached through copper-free click chemistry to polyP that had been derivatized with DBCO-PEG4-amine, thereby avoiding undesirable EDAC-mediated polymerization of peptides containing amines and carboxylates.
Conclusions: Optimized conditions for linking amine-containing compounds and peptides to polyP results in faster, more efficient labeling without polyP hydrolysis. Use of appropriate bridging molecules expands the coupling chemistries available for covalent polyP conjugates, facilitating the creation of novel, polyP-containing biomaterials for use in hemostasis, bone development, and other biomedical applications.