Introduction: Bone defect is one of the most common diseases in clinical department of orthopedic. It is badly in need of synthetic materials for the repair and reconstruction of bone defect[1]. Recently electrospinning is widely used for fabricating nanoscale fibers for tissue engineering due to its various advantages such as simplicity of process, high specific surface areas, tunable porosity[4]. In this study, PCL and PLLA were used as the main component for the nanofibers[2].
Materials and Methods: PLLA (MW 200 kDa), PCL (MW 80 kDa), HAP, DCM, DMF, dexamethasone, L-ascorbic acid, β-sodium glycerophosphate, α-MEM, CCK-8, Alkaline Phosphatase Assay Kit, alizarin red S, Cetylpyridinium chloride, Mouse Osteocalcin ELISA Kit.
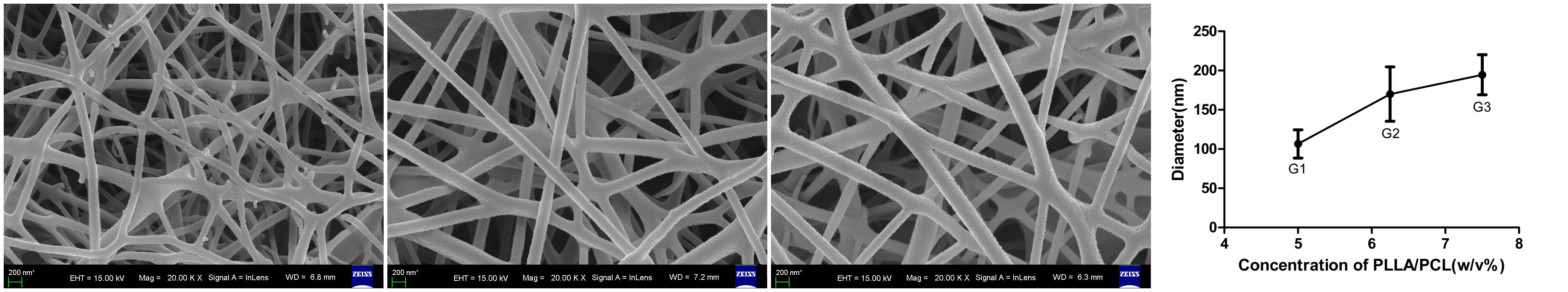
The scaffolds were obtained by electrospinning the mixed solution of PLLA/PCL/HAP[5]. The morphology of the scaffolds was investigated with SEM, while the diameter of the fibers, pore size were tested, respectively[6].
The in vitro bioactivity was assessed by CCK-8. The osteoblast mineralization were investigated the ALP activity and alizarin red S staining[3]. Osteocalcin (OCN) is another osteoblast marker that participate in controlling osteoblast function and bone extracellular matrix mineralization.
Results and Discussion: The morphology of the electrospun PLLA/PCL/HAP nanofibers was observed by SEM and the fiber diameters were showed by Figure 1. The pore size of the scaffold were 772.7±165.9 nm, 1167.2±168.9 nm, 1337.9±137.2 nm, respectively.
Pre-osteoblast MC3T3-E1 proliferation on scaffold was evaluated using CCK-8 test (Figure 2-left). ALP activity after MC3T3-E1 incubated on PLLA/PCL/HAP scaffold on 5, 7, and 9 days was showed in Figure 2-right.
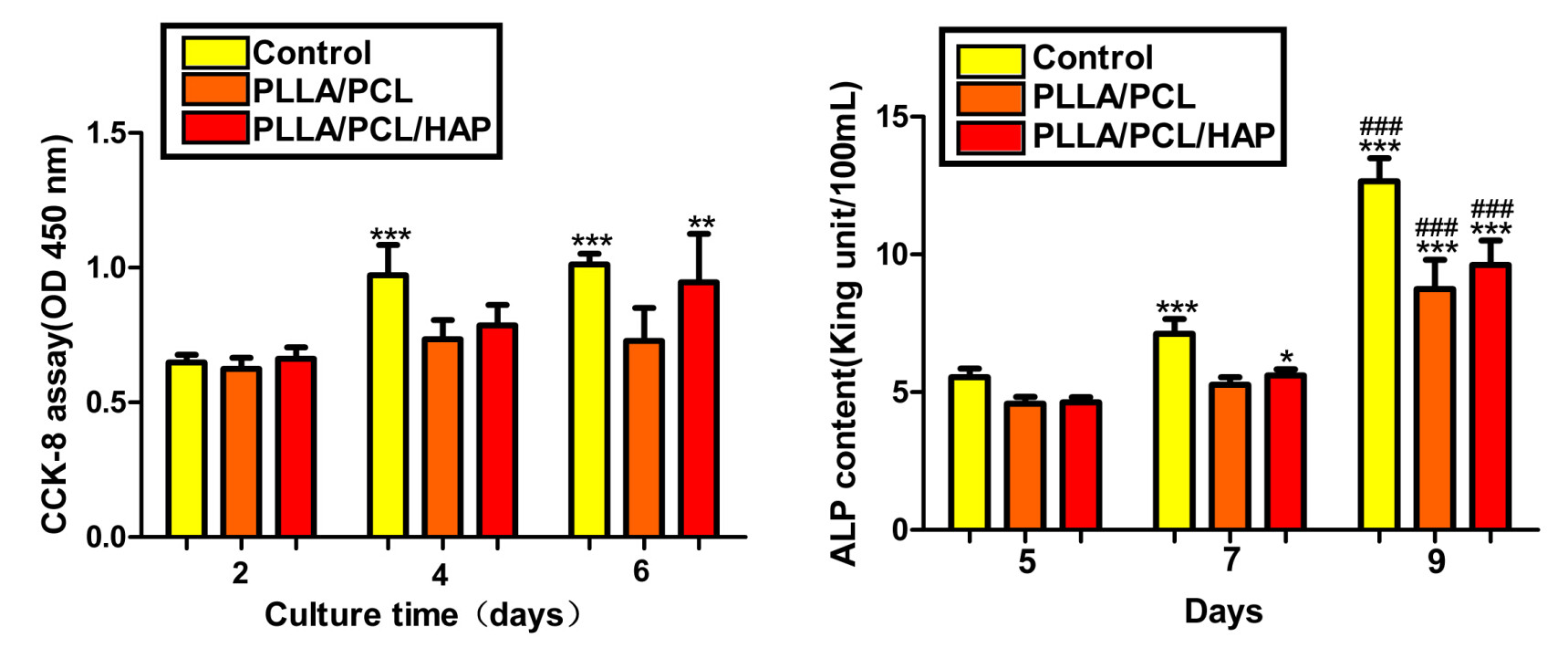
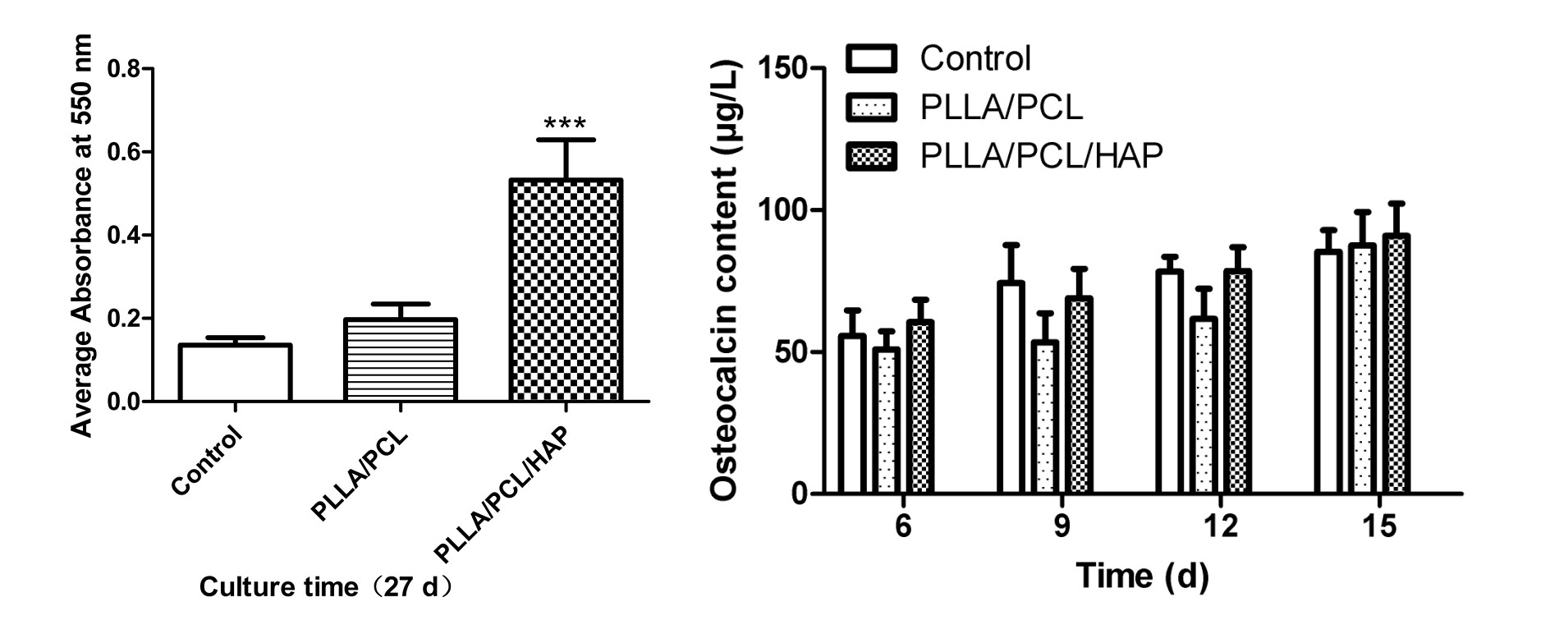
Figure 3-left shows the results of AR-S staining for the three groups after 27 days of culturing with MC3T3-E1. The secretion levels of osteocalcin of different osteogenic culture time were showed in Figure 3-right.
Conclusions: We designed PLLA/PCL nanofibrous scaffold containing HAP by electrospinning. Based on our experimental data, the composite scaffolds, PLLA/PCL/HAP especially, showed admirable bioactivity and promoted proliferation and osteogenic differentiation of MC3T3-E1 cells.
References:
[1] Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration
[2] Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres
[3] Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation
[4] Electrospun poly(L-lactic acid)/hydroxyapatite composite fibrous scaffolds for bone tissue engineering
[5] Electrospun polyurethane/hydroxyapatite bioactive scaffolds for bone tissue engineering: the role of solvent and hydroxyapatite particles
[6] Electrospun polyurethane/hydroxyapatite bioactive scaffolds for bone tissue engineering: the role of solvent and hydroxyapatite particles