Statement of Purpose: A surgical mesh is a woven or knitted polymer material implanted within the body to close an incision or hernia and reinforce the body tissue. After implantation, tissue integration and a foreign body response occur around the mesh[1]. These factors are influenced by structural characteristics of the mesh, such as pore size, and also impact postoperative complications associated with infection, hernia recurrence and chronic pain[2]. Therefore, it is clinically relevant to characterize pore size in surgical mesh removed (explanted) from patients, as mesh shrinkage during function in a physiological environment has been reported[3]. The purposes of this study were 1) to develop a new, high throughput method for accurate and efficient measurement of pore size in surgical mesh; and 2) to quantify changes in pore size by comparing measurements from explanted mesh and pristine mesh. It was hypothesized that pore sizes in explanted mesh will be significantly different than pristine mesh of the same type.
Methods: The new pore size characterization method involved three steps: image capture, automated image processing and final confirmation. First, calibrated digital images were captured using an optical light microscope with resolution of 0.124 pix/µm at 6x magnification and 0.243 pix/µm at 12x magnification. Second, local thresholding and automated noise reduction were used to obtain a binary image[4]. Third, only true pores were selected and visually confirmed by the user before final output of the pore size measurements. Images of a minimum of 1000 pores were acquired for each of four types of pristine surgical mesh, including Composix EX (Bard), Composix Kugel (Bard), Ultrapro (Ethicon) and Prolene Soft SPMXXL (Ethicon). The representative number of pores required for the data to converge within 5% of true pore size (TPS) was determined by averaging smaller sample sizes of pore measurements randomly selected from the 1000 pores.
Two types of explanted mesh (14 Composix Kugel and 5 Prolene Soft SPMXXL) were acquired after an average implantation time of 29∓22 months and 11∓8 months, respectively. Patients included 8 female and 11 male patients with an average age of 63∓13 years. Each mesh was fixed in formalin and digested free of tissues and then analyzed using the new pore size measurement method[5].
Results: The image characterization method was efficient, requiring 30 seconds for one image capture and 1 second for processing. Statistical analysis determined the effective number of pores (minimum of 40 pores per mesh type) required so that the average effective pore size was not significantly different from the TPS (i.e. within 5% of the TPS).

This analysis method is suitable for characterizing a broad range of pore sizes (~0.1 to 5 mm2). Preliminary data from a small sample size of explanted mesh confirm deviations in pore size relative to the pristine mesh (Figure 2 and Figure 3). The t-test showed significant difference between the mean value of pore size in pristine and explanted mesh (p < 0.01) for each set of pores for both types of mesh. Large pores in both types of mesh tended to shrink with trend toward enlargening of small pores, which indicated the mesh structure might change after implantation.
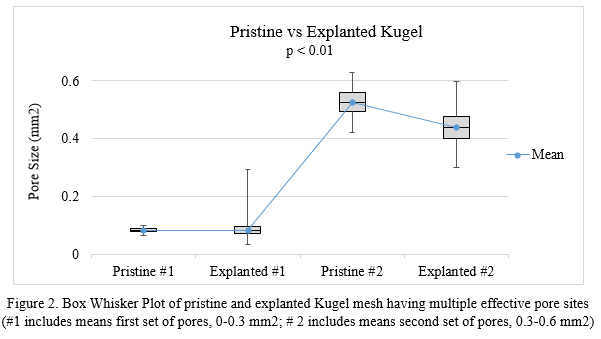
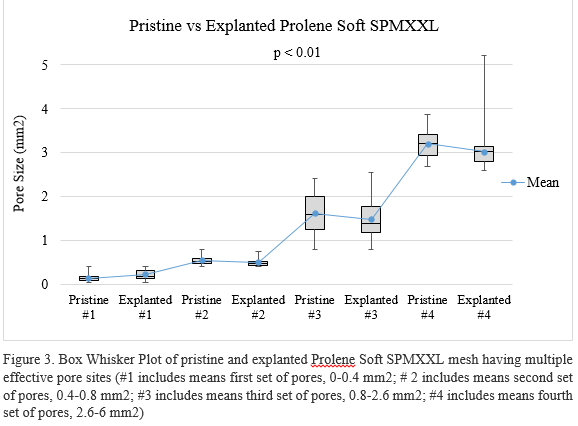
Conclusions: This pore size analysis optimized image capture for better distinction of mesh fibers and pores and faster image processing. Calculation of effective pore size based on measurement of 40 pores was within 5% of the true pore size. The high throughput method proved efficient for characterizing new and explanted surgical mesh. In ongoing work, this pore size analysis is part of a larger platform for assessment of clinical outcomes associated with structural characteristics of explanted surgical mesh.
References:
[1] A. Coda, R. Bendavid, F. Botto-Micc, et al. Structural alternations of prosthetic meshes in humans, Hernia, 7: 29-34 (2003)
[2] Klinge U, Klosterhalfen B. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia 16:251–258 (2012)
[3] TN Robinson, JH Clarke, J Schoen, MD Walsh. Major mesh-related complications following hernia repair. Surg Endosc, 19:1556-1560 (2005).
[4] Christian Wolf, Jean-Michel Jolion. Extraction and Recognition of Artifical Text in Multimedia Documents, Pattern Analysis and Applications, 6(4):309-326 (2003)
[5] Erin McClure Casey, Physical Characterization of Surgical Mesh after Function in Hernia Repair, Thesis, Clemson University (2015)