Introduction: Sarcomas such as osteosarcoma are treated with surgery and chemotherapy by anticancer drugs. The anticancer drugs cause various severe side effects, and prospective enough effects may not be obtained. So-called nano-particles smaller than cells have a property to enter cells and they are expected as drug delivery system (DDS). We heretofore reported biocompatibility and safety of the carbon nanotubes (CNT)[1],[2]. We report potential as DDS for chemotherapy to osteosarcoma cells and osteosarcoma model mice with CNTs.
Materials and Methods: The 143B cells (human osteosarcoma cell line) were seeded at 5.0×105 cells/10cm culture plate. After 24 hours, the culture medium was renewed to the medium contained 1 μg/ml or 10 μg/ml multi-walled CNT (MWCNT). Doxorubicin hydrochloride (DOX) (0.1 μM, 1.0 μM, 5.0 μM) was used as positive control. Each group was n=3. After more 24 hours, we observed the cells with light microscope and counted the number of 143B cells of each plate.
We made osteosarcoma model mice that injected 143B cells(1.0×106 cells) into the right tibia of male nude mice (BALB/ c-nu/nu). We mixed the MWCNTs and the 143B cells just before injection and the density of the MWCNTs were 1.0 μg/ml and 10 μg/ml. We added DOX just before injection as the positive control. Density of DOX were 1.0 μM and 5.0 μM. Each group were n=3. After 4 weeks, the size of the tumors in the legs were measured with CT and the number of lung metastases was evaluated histopathologically.
Results: In the light microscope images of the 143B cells that we added MWCNTs before 24 hours, the MWCNTs were taken in the cells. In the MWCNT 10 μg/ml group, much MWCNTs were taken in the 143B cells than the MWCNT 1 μg/ml group. The cell number after 24 hours culture was 23.3×105 cells/plate in control, 10.3×105 cells/plate in DOX 0.1 μM group,5.6×105 cells/plate in 1.0 μM group, 2.8×105 cells/plate in 5.0 μM group,21.3×105 cells/plate MWCNT 1 μg/ml group and 16.3×105 cells/plate MWCNT 10 μg/ml group.
The mean tumor size were 1729 mm3 in the control group, 1399 mm3 in the MWCNT 1.0 μg/ml group and 394 mm3 in the MWCNT 10 μg/ml group. MWCNTs inhibited the growth of osteosarcomas in concentration-dependency.
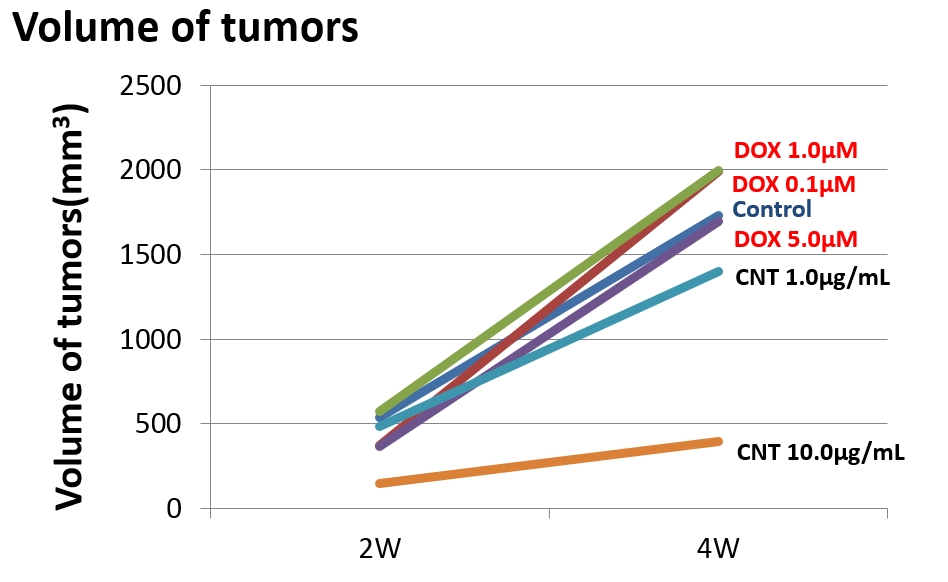
And then, the mean metastasis number in one sections of the lung tissue was 5.5 in the control, 2.2 in the MWCNT 1.0 μg/ml group and 0.7 in the MWCNT 10 μg/ml group. In the MWCNT 10 μg/ml group, lung metastases were inhibited significantly.
Discussion: When the MWCNTs are added to the osteosarcoma cell line; 143B cells, the MWCNTs are taken into the cells and inhibited a cellular proliferation in concentration-dependency. And MWCNTs inhibited the growth of tumors and lung metastases in the osteosarcoma model mice.
Conclusion: By adhering anticancer drugs to the MWCNTs, we expect to improve invasive efficiency to sarcoma cells of the anticancer drugs, to enhance the chemotherapeutic effect and to reduce the chemotherapeutic side effects.
This work was supported by JSPS KAKENHI Grant Number 26861180.
References:
[1] Narita N. et al., Nano Lett. 9:1406-13, 2009
[2] Hara K, et al., Materials Today. 14:434-440, 2011