Introduction: Diamond nanoparticles (DNPs) are considered as a highly biocompatible material for biomedical applications. In our earlier studies, nanocrystalline diamond films proved as excellent substrates for the adhesion, growth and osteogenic differentiation of human-bone derived cells, particularly after their doping with boron[1] or termination with oxygen[2]. In addition, diamond nanoparticles could be added into nanofibrous polymeric scaffolds in order to improve their mechanical properties and bioactivity for bone tissue engineering. Thus, composite poly(L-lactide) (PLLA) nanofibrous membranes with six different concentrations of DNPs were prepared, and the adhesion, growth and osteogenic differentiation of human osteoblast-like MG-63 and Saos-2 cells were studied on these matrices.
Materials and Methods: Five grams of PLLA were dissolved in 100 ml of chloroform, and DNPs (NanoAmando, Nanocarbon Research Institute Co., Ltd., Japan) were added in 6 concentrations ranging from 0.021875 to 0.7 g/100 ml of PLLA solution, i.e. from 0.38 to 12.28 wt.% in dry PLLA. Nanofibrous membranes were then prepared by needle-less electrospinning. The cell growth was estimated using XTT test, measuring the activity of mitochondrial enzymes. The concentrations of focal adhesion proteins talin and vinculin, and also of osteogenic proteins collagen I, alkaline phosphatase, osteopontin and osteocalcin, were measured in cell homogenates by an enzyme linked-immunosorbent assay (ELISA) on day 7 after seeding. The expression of mRNA for these proteins was measured by real-time PCR on day 14 after seeding.
Results and Discussion: The activity of mitochondrial enzymes decreased with increasing nanoparticle concentration in both MG-63 and Saos-2 cells (Fig. 1).
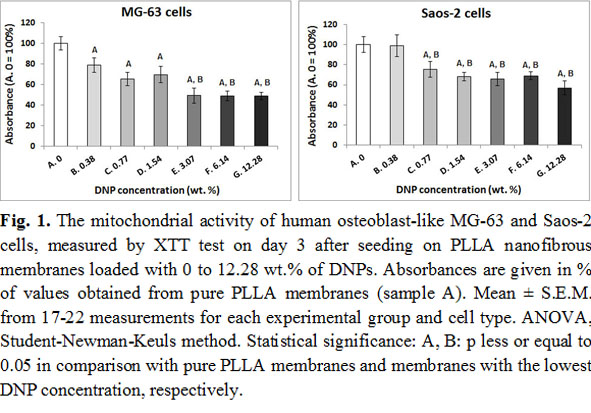
The concentration of focal adhesion and osteogenic proteins, measured on day 7 at the protein level, was similar in cells on pure PLLA scaffolds and scaffolds with all concentrations with DNPs, except of osteocalcin in MG-63 cells, which decreased with increasing nanoparticle concentration. On day 14 after seeding, the mRNA expression of talin and vinculin, as well as of collagen I, alkaline phosphatase and osteopontin showed a general tendency to decrease with increasing DNP concentration (Fig. 2).
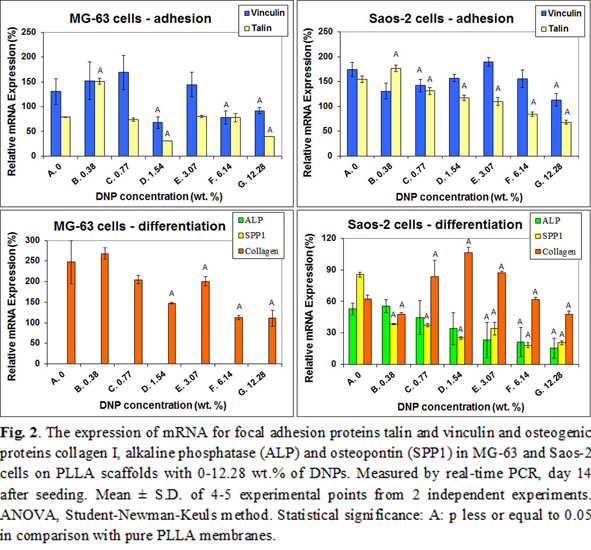
On the other hand, the addition of DNPs to PLGA nanofibers in our earlier studies supported the growth of MG-63 cells[3] and human bone marrow mesenchymal stem cells[4], even in a higher concentration (23 wt.%). This disproportion could be explained by different origin and physicochemical properties of DNPs used for PLLA (detonation diamond) and for PLGA (DNPs prepared by a RF-PACVD method[5]). The DNPs used for PLLA scaffolds were hydrogen-terminated and hydrophobic, which markedly increased the hydrophobicity of the PLLA-DNP scaffolds, making them less appropriate for the colonization with cells. A direct cytotoxicity of DNPs cannot also be excluded. DNPs can damage cells by oxidative mechanisms and by an increased influx of Na+ ions into cells (for a review, see[4]).
Conclusion: Addition of diamond nanoparticles produced by detonation and terminated with H into nanofibrous scaffolds had negative effects on the adhesion, growth and osteogenic differentiation of human bone-derived cells, while the effects of diamond nanoparticles produced by RF-PACVD method were beneficial. Thus, the mode of preparation and surface properties of diamond nanoparticles could be important for their biocompatibility and for their use as components of biomaterials.
Supported by the Grant Agency of the Czech Republic (grants No. P108/12/1168 and 14-04790S) and the Ministry of Health of the Czech Republic (grant No. 15-32497A).
References:
[1] Grausova L et al.: PLoS One 6(6):e20943, 2011
[2] Liskova J et al.: Int J Nanomedicine 10:869-84, 2015
[3] Parizek M et al.: Int J Nanomedicine 7:1931-51, 2012
[4] Brady MA et al.: J Nanosci Nanotechnol 15:1060-9, 2015
[5] Mitura S et al.: J AMME 16:9-16, 2006