Introduction: Rupture of atherosclerotic plaques causes acute clinical events, such as ischemic stroke and myocardial infarct. Information about cellular activation appears to be highly relevant for predicting plaque evolution to rupture[1]. P-selectin expressed by activated platelets and endothelial cells is upregulated in unstable plaques[2]. Nano and microsystems targeted towards P-selectin have been developed for the diagnosis of cardiovascular diseases. In particular, fucoidan-based systems were shown to detect platelet and endothelial activation in vivo[3]-[5]. However prior to in vivo evaluation, there is a need to analyze in vitro the interactions of such systems with their molecular targets. In addition to published techniques, such as flow cytometry[5] and interaction studies with P-selectin at low shear stress[6], we developed a method to visualize and quantify the affinity of fucoidan-coated microparticles for recombinant P-selectin and for human activated platelet aggregates expressing P-selectin in arterial flow conditions.
Materials and Methods: Fluorescent polymer microparticles (MPs) were synthesized by anionic emulsion polymerization and tested as model systems. Fucoidan coated MPs (Fuco-MPs) and control MPs (Control-MPs) were injected in channels of Vena8 Fluoro+ chambers (Cellix Ltd) coated with either recombinant P-, E- or L-selectin. ExiGo pump (Cellix Ltd) was used to generate arterial flow conditions (shear stress 67.5 dyne/cm2). MPs adhesion was visualized and quantified in real time under fluorescence microscopy. A second set of experiments consisted first in injecting human whole blood into collagen coated channels to induce platelets activation and aggregation. Fuco-MPs or Control-MPs were then injected in the channels and their adhesion onto platelet aggregates was recorded.
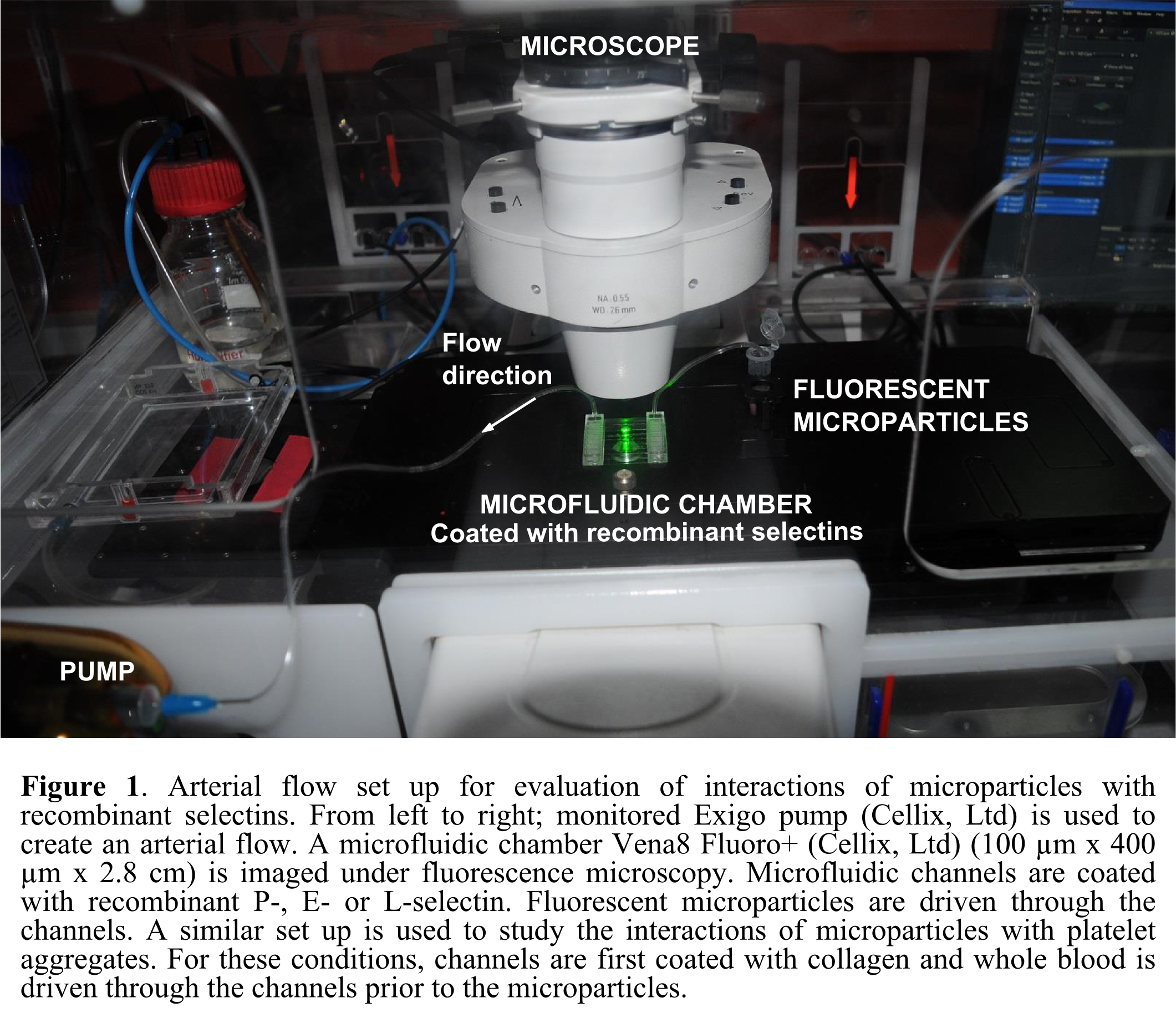
Results and Discussion: Fuco-MPs bound significantly more to the P-selectin coating than Control-MPs (p<0.01). Fuco-MPs adhered immediately after injection in a P-selectin dose-dependent manner. Furthermore, their adhesion to L- and E-selectins was significantly lower than to P-selectin (p<0.01). These results are encouraging as regards to the sensitivity and selectivity of Fuco-MPs, confirming fucoidan potential for active targeting. Moreover, only MPs coated with fucoidan accumulated at the surface of platelet aggregates. Fuco-MPs appear promising for therapeutic and diagnostic targeted delivery.
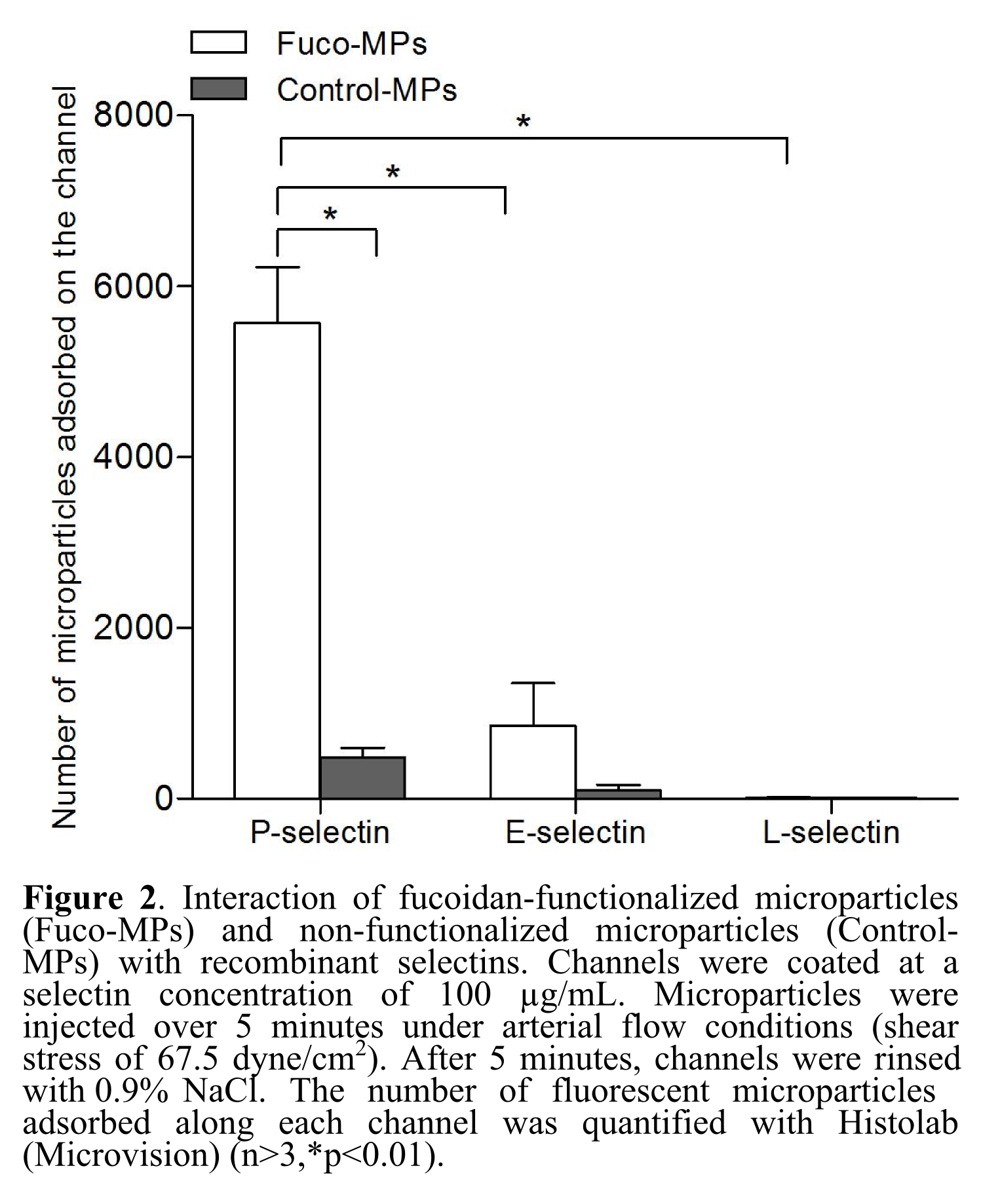
Conclusion: A model was set up to mimic microparticles adhesion on P-selectin that is expressed on platelet aggregates occurring in thrombus formation. It could be used routinely to validate the targeting efficacy of other microsystems before their in vivo evaluation. Future developments include the implementation of a layer of activated endothelial cells. By offering a robust and tunable model of vascular wall lesions, this work participates in the development of personalized therapy and diagnosis in the cardiovascular field.
NanoAthero FP7-NMP-2012-LARGE-6-309820; ANR-13-LAB1-0005-01 "FucoChem"
References:
[1] P. Libby, M. DiCarli, R. Weissleder, The vascular biology of atherosclerosis and imaging targets. J. Nucl. Med. 51 Suppl 1 (2010) 33S-37S
[2] K. Ley, The role of selectins in inflammation and disease. Trends Mol. Med. 9(6) (2003) 263-268
[3] F. Rouzet, L. Bachelet-Violette, J.M. Alsac, M. Suzuki, A. Meulemans, L. Louedec, A. Petiet, M. Jandrot-Perrus, F. Chaubet, J.B. Michel, D. Le Guludec, D. Letourneur, Radiolabeled fucoidan as a p-selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J. Nucl. Med. 52(9) (2011) 1433-1440
[4] T. Bonnard, G. Yang, A. Petiet, V. Ollivier, O. Haddad, D. Arnaud, L. Louedec, L. Bachelet-Violette, S.M. Derkaoui, D. Letourneur, C. Chauvierre, C. Le Visage, Abdominal aortic aneurysms targeted by functionalized polysaccharide microparticles: a new tool for SPECT imaging. Theranostics 4(6) (2014) 592-603
[5] T. Bonnard, J.M. Serfaty, C. Journe, B. Ho Tin Noe, D. Arnaud, L. Louedec, S.M. Derkaoui, D. Letourneur, C. Chauvierre, C. Le Visage, Leukocyte mimetic polysaccharide microparticles tracked in vivo on activated endothelium and in abdominal aortic aneurysm. Acta Biomater. 10(8) (2014) 3535-3545
[6] B.P. Davidson, B.A. Kaufmann, J.T. Belcik, A. Xie, Y. Qi, J.R. Lindner, Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J. Am. Coll. Cardiol. 60(17) (2012) 1690-1697