Introduction: Silated hydroxypropylmethylcellulose (Si-HPMC) is an injectable and self-setting hydrogel used as a three-dimensional synthetic matrix for tissue engineering. Si-HPMC is a gel based on a derivate from HPMC, a cellulose derivative polymer. This biocompatible polysaccharide forms a covalently crosslinked hydrogel due to silanol condensation[1]-[3].
Materials and Methods: A thin layer of Si-HPMC hydrogel disks was transferred to a freshly cleaved mica surface. The Si-HPMC hydrogel was applied to the top surface of the mica.
Images of Si-HPMC hydrogel were obtained by atomic force microscopy (AFM) (Bruker© Icon, 2010). During 24 hours, Si-HPMC hydrogel surface was observed. The thin hydrogel layer adhering to the mica surface was scanned with the AFM operating in tapping mode using an etched single crystal silicon probe. The drive frequency, amplitude, gains, and amplitude set point ratio were adjusted to give height and phase images with the clearest image details.
AFM (height and phase shift) images analysis and examination in Fourier transformed operation mode were performed with the software NanoScope Analysis 1.30 (Bruker Corporation©, 2011).
Results and Discussion: Polymerization and cross-linking of the polymer begin in the first seconds and continue during the whole time of observation.
Height and phase shift images reflect the surface topography and relative mechanical responses of the sample, respectively. Images demonstrated a network-like structure with pores. The gels consisted of branched structures where the branch points may be interpreted as points where junctions between silanol cross-linked chains occur. The nanosized pore range from approximately 10-20 nm. Some of the pores were interconnected.
Surface topography and relative mechanical responses regularly changes over time (fig. 1 and fig. 2).
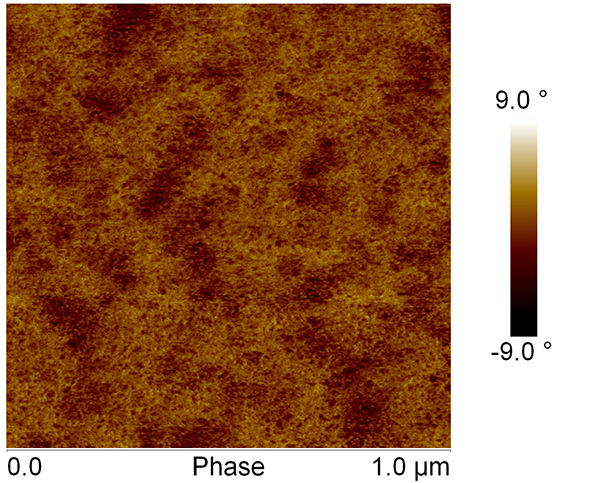
Fig. 1: AFM phase image of Si-HPMC hydrogel layer, after 30 minutes of AFM scanning. Scale is 1µm.
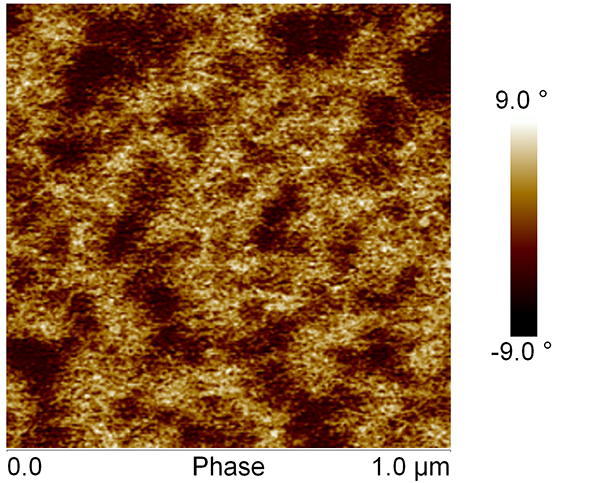
Fig. 2: AFM phase image of Si-HPMC hydrogel layer, after 7 hours of AFM scanning. Scale is 1µm.
The network becomes more complex with time.
Examination of AFM images, in Fourier transformed operation mode of tapping mode-AFM, revealed that Si-HPMC hydrogel is characterized by an isotropic structure.
Conclusion: These images revealed for the first time how the network is organized within Si-HPMC hydrogel and demonstrated that Si-HPMC hydrogel is characterized by an isotropic structure.
References:
[1] Fatimi A, Tassin JF, Quillard S, Axelos MAV, Weiss P. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials 2008;29:533‑43.
[2] Fatimi A, Tassin J-F, Turczyn R, Axelos MAV, Weiss P. Gelation studies of a cellulose-based biohydrogel: the influence of pH, temperature and sterilization. Acta Biomater 2009;5:3423‑32.
[3] Bourges X, Weiss P, Daculsi G, Legeay G. Synthesis and general properties of silated-hydroxypropyl methylcellulose in prospect of biomedical use. Adv Colloid Interface Sci 2002;99:215‑28.