Dendrimer-based sentinel lymph node imaging
-
1
Osaka Prefecture University, Dept. Applied Chemistry, Japan
-
2
Hokkaido University, Faculty of Pharmaceutical Sciences, Japan
-
3
Hamamatsu University School of Medicine, Medical Photonics Research Center, Japan
Introduction: Dendrimers have highly controllable size and surface properties, which are quite different from linear polymers. Dendrimers are a potent imaging probe, because various kinds of detectable probes can be conjugated to terminal functional groups of a single dendrimer molecule. Previously, we have prepared dendrimers conjugating gadolinium-chelates and radioactive indium-chelates for magnetic resonance imaging (MRI) and singly photon emission computed tomography (SPECT)[1],[2]. A sentinel lymph node is the first lymph node to which cancer cells are spread. Detection of sentinel lymph node is important for cancer therapy and diagnosis. However, there are only a few reports on dendrimer-based imaging agents for sentinel lymph node imaging. In this study, twelve radiolabeled dendrimers with different sizes and different surfaces were prepared as dendrimer imaging agents for SPECT imaging. These dendrimer imaging agents were intradermally injected into rats, followed by the biodistribution analysis and the SPECT imaging of lymph nodes, to obtain knowledge on the optimized dendrimer structure for sentinel lymph node imaging.
Results and Discussion: Several chelate molecules (DTPA) were conjugated to amino-terminal polyamidoamine (PAMAM) dendrimers of generation (G) 2, 4, 6 and 8 to obtain cationic dendrimers. The residual terminal amino groups were acetylated (Ac) and carboxylated to obtain nonionic and anionic dendrimers, respectively. These compounds were characterized by NMR, UV-Vis spectrometry and DLS. The size increased with increasing the dendrimer generation, and the zeta potential was largely affected by the terminal group. These dendrimers were labeled with radioactive 111In3+ ions, followed by the intradermal injection into rats. The biodistribution after 1, 6 and 24 h was analyzed. Small dendrimers (G2 and G4) were mostly localized in the kidney. Cationic dendrimers of larger than G4 retained at the injection site. Acetylated dendrimers were flowed into blood. Large and anionic dendrimers (G8-COOH, G6-COOH and G4-COOH) were efficiently distributed in the sentinel lymph node (Fig. 1). The SPECT imaging by injecting G6-COOH succeeded detection of the sentinel lymph nodes (Fig. 2). Fluorescent dye-labeled dendrimers were also prepared, and fluorescent imaging of thin section of the sentinel lymph node was performed. Interestingly, these dendrimers were not recognized by immune cells such as macrophages and T-cells. Thus, it is likely that the medium flow speed of the carboxyl-dendrimers of larger than G4 affected the accumulation of the sentinel lymph node.
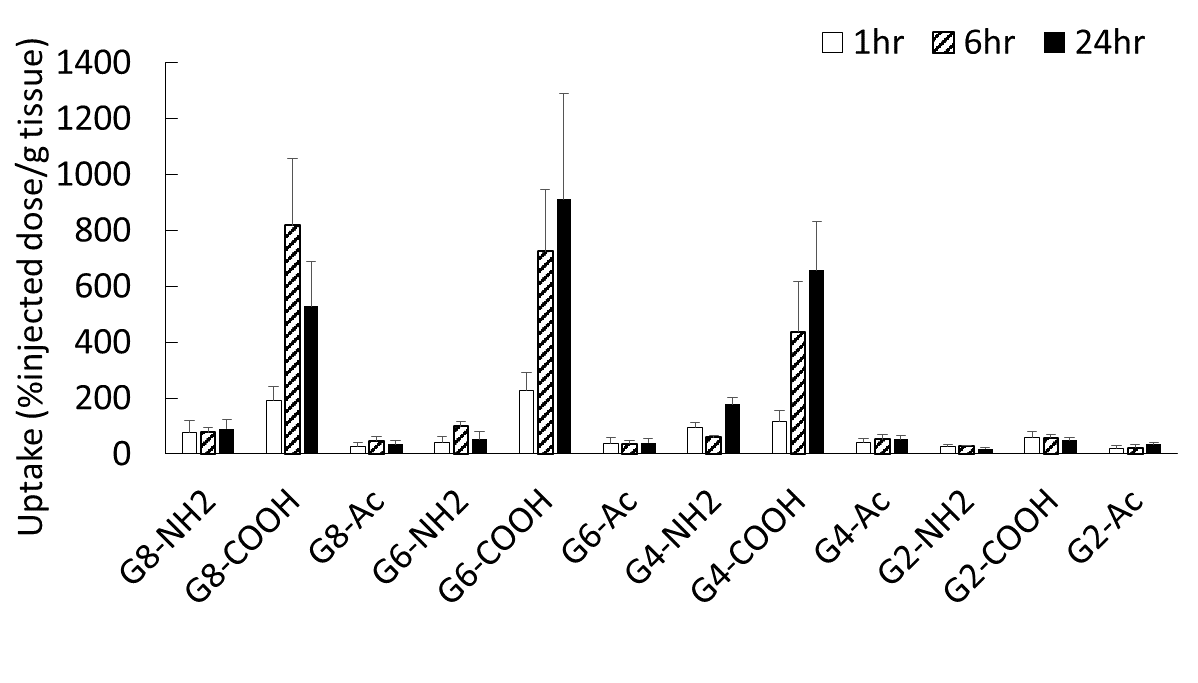
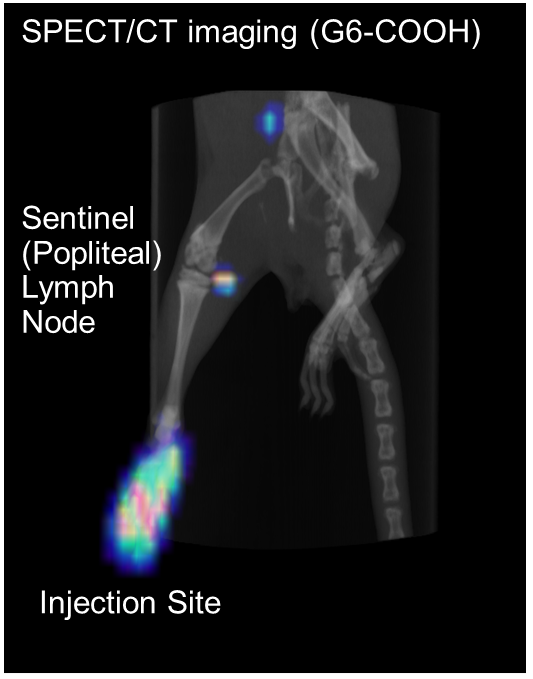
Conclusion: We succeeded the sentinel lymph node imaging by using carboxy-terminated dendrimers with larger than G4 (larger than 6 nm). These dendrimers are useful for sentinel lymph node biopsy.
References:
[1] C. Kojima, B. Turkbey, M. Ogawa, et al, Nanomedicine, 7, 1001-8 (2011).
[2] C. Kojima, Y. Niki, M. Ogawa, R. Makiura and Y. Magata, Int J Pharm, 476, 164-8 (2014).
Keywords:
Molecular Imaging,
in vivo,
polymer,
surface property
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Imaging with biomaterials
Citation:
Kojima
C,
Niki
Y,
Ogawa
M and
Magata
Y
(2016). Dendrimer-based sentinel lymph node imaging.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01886
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Chie Kojima, Osaka Prefecture University, Dept. Applied Chemistry, Sakai, Japan, Email1
Dr. Yuichiro Niki, Osaka Prefecture University, Dept. Applied Chemistry, Sakai, Japan, Email2
Dr. Mikako Ogawa, Hokkaido University, Faculty of Pharmaceutical Sciences, Sapporo, Japan, Email3
Dr. Yasuhiro Magata, Hamamatsu University School of Medicine, Medical Photonics Research Center, Hamamatsu, Japan, Email4