Unravelling the binding energetics of a synthetic polymeric heparin antidote with heparin using isothermal titration calorimetry
-
1
University of British Columbia, Pathology and laboratory Medicine, Canada
-
2
University of British Columbia, Department of Chemical and Biological Engineering, Canada
-
3
University of British Columbia, Chemistry, Canada
Background and Objective: To avert haemorrhage associated with heparin therapy, antidotes are required. Protamine sulphate (PS), a cationic peptide is used to reverse unfractionated heparins (UFH). PS electrostatically interacts with heparins to generate complexes devoid of anticoagulation. However, PS partially neutralizes anticoagulation of low molecular weight heparins (LMWHs) and is ineffective against fondaparinux. Understanding this clinical need, we developed a Universal Heparin Reversal Agent (UHRA) capable of reversing anticoagulation of all clinically available parenteral anticoagulants. UHRA has three components: hyperbranched polyglycerol core comprising heparin binding groups and methoxy polyethylene glycol (mPEG) chains emanating from the core. Using Isothermal titration calorimetry (ITC), we observed that both PS and UHRA bind to UFH, LMWHs and fondaparinux with micromolar dissociation constant. This raises an important question: why UHRA is an efficient heparin antidote with minimal non-specific protein binding, while PS fails to reverse the anticoagulation effects of LMWHs and fondaparinux and binds to proteins in competitive media such as human blood. We hypothesize that the presence, density and length of mPEG chains in UHRA is crucial to attain maximum efficacy and minimal toxicity. In this study, we use ITC to demonstrate the structural superiority of UHRA in comparison to PS.
Methods: UHRA was synthesized by anionic ring opening polymerization of glycidol and mPEG, post-modified with multifunctional tertiary amines to generate cationic groups (Figure 1). We synthesized UHRA with no mPEG chains (N-UHRA) to evaluate its crucial role in the design of UHRA. ITC experiments were performed on VP-ITC microcalorimeter. We titrated UHRA, N-UHRA and PS against UFH at varying salt concentrations. Activated partial thromboplastin time assay (aPTT) was performed in human platelet poor plasma (PPP) and anticoagulated human PPP respectively to assess biocompatibility and neutralization capability of antidotes.
Results and Discussion: As anticipated, the binding affinity of antidotes to UFH decreased with increase in salt content in the buffer solution (Figure 1).
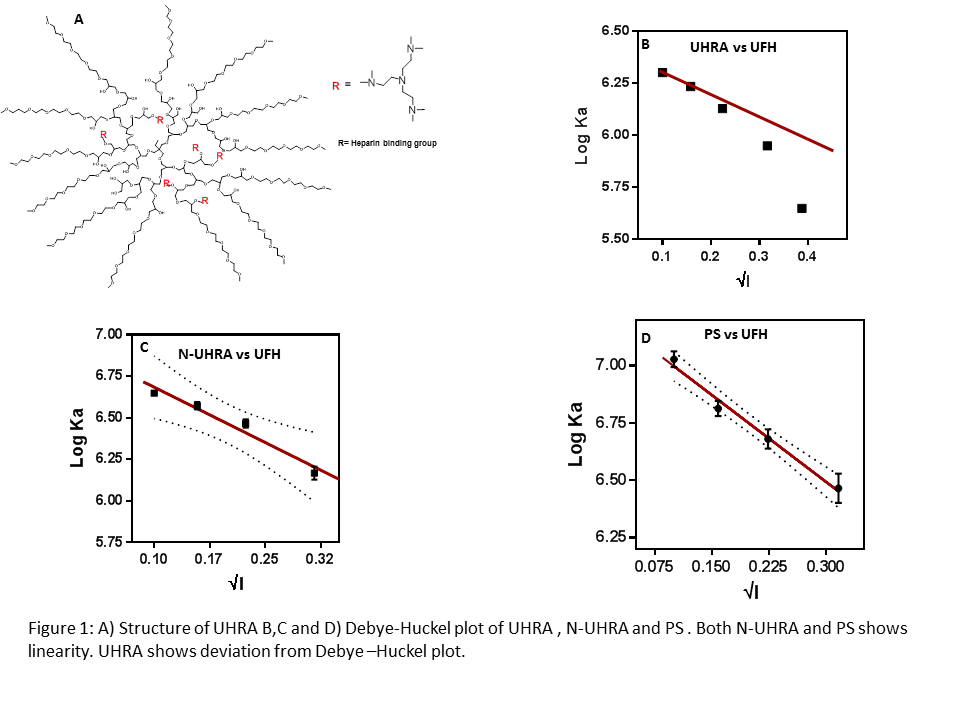
Debye-huckel plot of association constant versus salt concentration showed linear relationship for both N-UHRA and PS. However, a deviation from Debye-Huckel plot (non-linearity) was observed in the binding between UFH and UHRA. The binding of UHRA to UFH is enthalpy driven, but the presence of mPEG chains acts as entropic bumper, reducing the binding affinity rapidly as the salt concentration increases in comparison to N-UHRA or protamine providing the required selectivity. We observed that the binding affinity of UHRA to UFH decrease with increase in temperature, and the complex formation becomes more exothermic. The linearity of enthalpy-entropy compensation plot ,slope close to 1.0 reveals an exact compensation of enthalpy by entropy. The intrinsic anticoagulation effect exhibited by UHRA was mild compared to N-UHRA and PS. Also, UHRA completely neutralized UFH anticoagulation over a broad concentration ranging from 12.5 µg/mL to 200 µg/mL. On the other hand, protamine and N-UHRA failed to completely reverse anticoagulation effects of UFH and showed anticoagulation effect above 100 µg/mL (Figure 2).
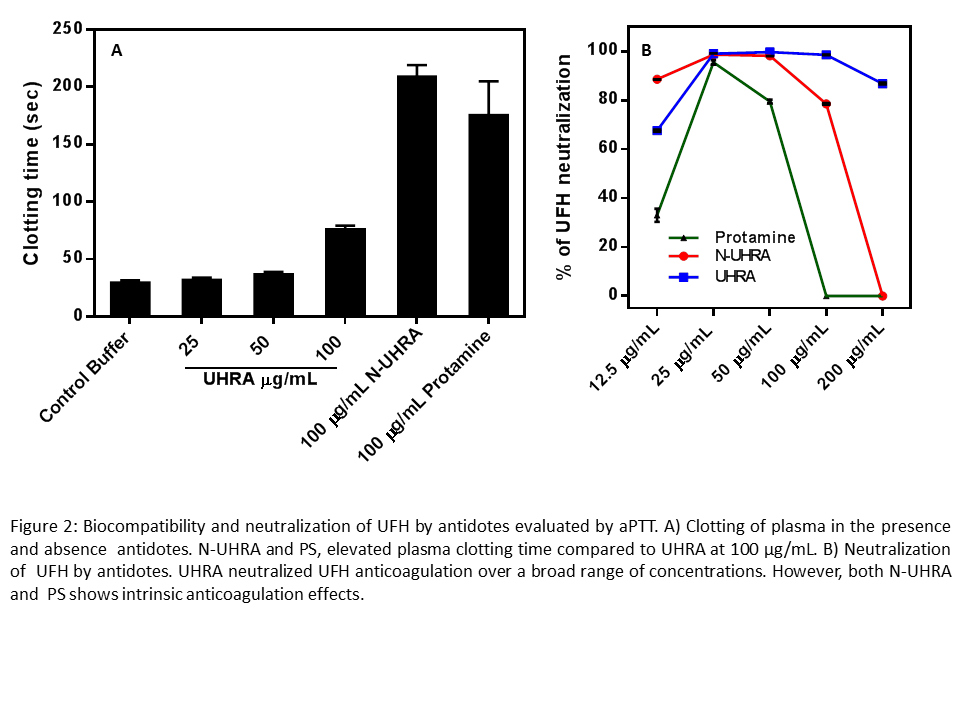
Conclusions: The nonspecific interactions of N-UHRA and PS to plasma proteins could be responsible for their intrinsic anticoagulation. This study shows that mPEG brush layer (entropic barrier) could reduce interactions of UHRA with plasma proteins and provide selectivity in binding. However, the entropic barrier of UHRA is overwhelmed in the presence of highly anionic, molecules like heparins making UHRA an ideal heparin antidote.
Canadian Institutes of Health Research (CIHR); Natural Science and Engineering Research Council (NSERC)
Keywords:
Mechanism,
nanoparticle,
Polymeric material,
biomacromolecule
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for cardiovascular applications, vascular grafts and embolic devices
Citation:
Kalathottukaren
M,
Srinivas
A,
Rajesh
S,
Louise
CA,
Haynes
C and
Kizhakkedathu
J
(2016). Unravelling the binding energetics of a synthetic polymeric heparin antidote with heparin using isothermal titration calorimetry.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01840
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Manu Thomas Kalathottukaren, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email1
Dr. Shenoi Rajesh, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email2
Dr. Creagh A Louise, University of British Columbia, Department of Chemical and Biological Engineering, Vancouver, BC, Canada, Email3
Dr. Charles Haynes, University of British Columbia, Department of Chemical and Biological Engineering, Vancouver, BC, Canada, Email4
Dr. Jayachandran Kizhakkedathu, University of British Columbia, Pathology and laboratory Medicine, Vancouver, BC, Canada, Email5