Proliferation and osteogenic differentiation of amniotic fluid derived stem cells cultured on biomaterials having nano segments and optimal elasticity
-
1
National Central University, Chemical and Materials Engineering, Taiwan
-
2
King Saud University, Department of Botany and Microbiology, Saudi Arabia
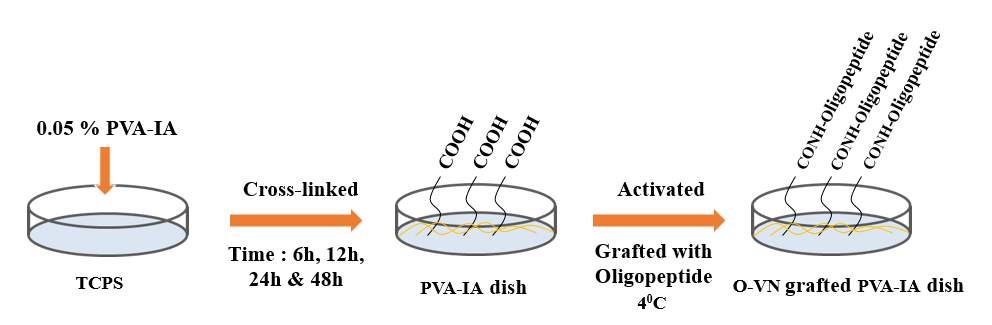
Human amniotic fluid-derived stem cells (hAFSCs) are pluripotent fetal cells capable of differentiating into multiple lineages, including representatives of the three embryonic germ layers. AFSCs may become a more suitable source of stem cells in regenerative medicine and tissue engineering. However, stem cell characteristics, such as proper differentiation and maintenance of pluripotency, are regulated not only by the stem cells themselves but also by their microenvironment[1]. Furthermore, physical characteristics of cell culture substrates such as substrate elasticity may influence the fate of stem cell differentiation[2],[3]. We investigated efficiency of osteogenic differentiation of hAFSCs cultured on cell culture substrates which have different elasticities and are immobilized with extracellular matrix-derived oligopeptides. We prepared dishes coated with polyvinylalcohol-co-itaconic acid (PVA-IA) films having different elasticities by controlling the crosslinking time in crosslinking solution that contains glutaraldehyde. The PVA-IA dishes were grafted with ECM-derived oligopeptides through N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) chemistry in an aqueous solution.
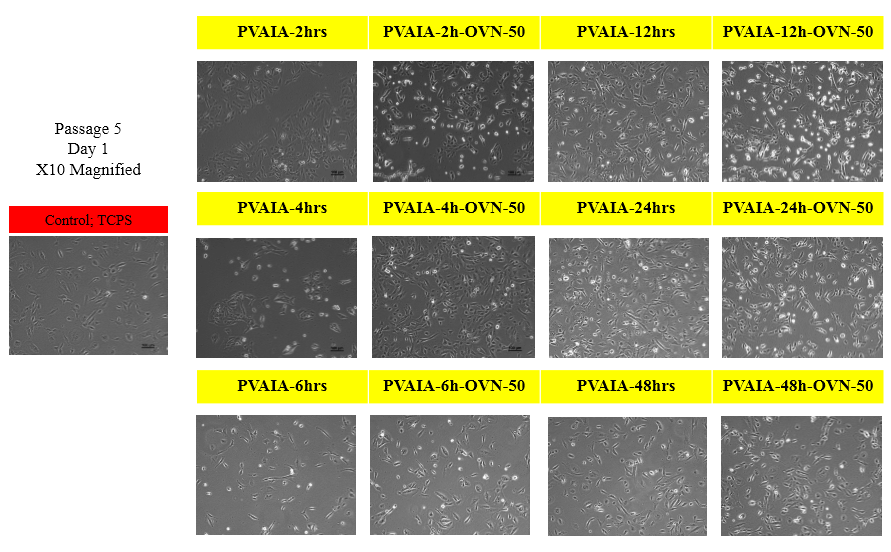
Pluripotent gene expressions (Nanog, Oct4 and Sox2) were evaluated by the qRT-PCR measurements. We found that there is an optimal elasticity of cell culture matrix to keep pluripotency of AFSCs for their culture. To characterize osteogenic differentiation of hAFSCs, we checked alkaline phosphatase (ALP) activity, alizarin Red S staining and von Kossa staining after two weeks and four weeks of culture in induction media, respectively.
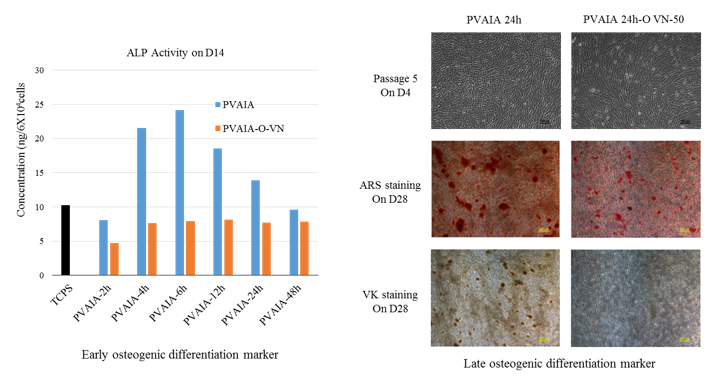
It is suggested that physical cues such as stiffness of culture materials as well as biological cues of extracellular matrix components can guide and decide differentiation of hAFSCs into osteoblasts.
References:
[1] Higuchi, A., Ling, Q. D., Hsu, S. T., Umezawa, A., Chem. Rev. 2012, 112, 4507−4540
[2] Kolhar, P., Kotamraju, V. R., Hikita, S. T., Clegg, D. O., Ruoslahti, E., J. Biotechnol. 2010, 146, 143–146
[3] Engler, A.J., et al., Matrix elasticity directs stem cell lineage specification. Cell 2006, 126(4), 677-689
Keywords:
Cell Differentiation,
Cell Proliferation,
stem cell,
biomaterial
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials to modulate biological processes involved in host response
Citation:
Muduli
S,
Lin
H,
Higuchi
A and
Chen
Y
(2016). Proliferation and osteogenic differentiation of amniotic fluid derived stem cells cultured on biomaterials having nano segments and optimal elasticity.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01606
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Saradaprasan Muduli, National Central University, Chemical and Materials Engineering, Jhongli City, Taiwan, saradaprasan.muduli@gmail.com
Dr. Hong Ren Lin, National Central University, Chemical and Materials Engineering, Jhongli City, Taiwan, frogl0612@gmail.com
Dr. Akon Higuchi, National Central University, Chemical and Materials Engineering, Jhongli City, Taiwan, akon.higuchi@gmail.com
Dr. Yen Ming Chen, National Central University, Chemical and Materials Engineering, Jhongli City, Taiwan, lebronai@hotmail.com