Introduction: Polypeptides form higher-order structures assembled by secondary structures, which affect their stability and function. Cisplatin-loaded micelles, which are nanocarrier-based drug delivery systems undergoing human clinical trials, are prepared via self-assembly of poly(ethylene glycol)-b-poly(L-glutamic acid) [PEG-P(Glu)] by reaction with anticancer platinum drug cisplatin[1]. Here, we studied the drug delivery performance of cisplatin-loaded micelles in relation to the higher-order structure in the core-forming P(Glu) blocks.
Materials and Methods: Cisplatin was reacted in water with a series of PEG-P(Glu) having different ability to adopt secondary structures in the P(Glu) block: PEG-P(L-Glu) and PEG-P(D-Glu), which can adopt α-helix, and PEG-P(D,L-Glu), which adopts random conformation, to obtain L-, D- and D,L-micelles, respectively. The diameter of the micelles and their core morphology were examined by dynamic light scattering and transmission electron microscopy, respectively. The secondary and higher-order structure of the P(Glu) blocks were analyzed by circular dichroism (CD) spectroscopy and small angle X-ray scattering (SAXS). Drug release was studied by dialysis in PBS (150 mM NaCl; pH 7.4) in combination with inductively coupled plasma mass spectrometry (ICP-MS) for Pt quantification. Micelle disassembly was followed by static light scattering and sedimentation velocity method. Mice (BALB/c) bearing s.c. xenografts of human pancreatic adenocarcinoma (BxPC3) were used for animal experiments. Biodistribution was examined by collecting the plasma, tumor, liver, spleen and kidney after i.v. injection of the micelles, followed by ICP-MS analysis. Antitumor efficacy was tested by following the tumor volume after injecting the micelles i.v. (4 mg/kg) three times every other day.
Results and Discussion: All the micelles produced in a narrow size distribution (~25 nm) with a spherical core. L- and D-micelles had α-helices in 70% of the P(Glu) blocks, as confirmed by mean residue ellipticity at 222 nm on the CD spectra. L-micelles was further analyzed by SAXS, which gave a diffraction peak (q = 5 nm-1) corresponding to bundled assembly of α-helices[2].
In PBS, chloride ions induced cisplatin release and micelle disassembly, which proceeded by releasing the unimers and dimers of the cisplatin-conjugated polymers. In this process, L- and D-micelles disintegrated slowly at constant rate over 96 h, whereas D,L-micelles broke up rapidly after 10 h, showing the stabilizing effect of the α-helix bundle. After i.v. injection, L- and D-micelles showed prolonged blood circulation, whereas D,L-micelles were uptaken by liver and spleen after 8 h and decreased their plasma concentration (Fig. 1A). This increased bioavailability of L- and D-micelles led to augmented cisplatin delivery to tumor tissues via enhanced permeability and retention effect (Fig. 1B)[3], resulting in suppressive antitumor efficacy compared to D,L-micelles, indicating the major role of the α-helix bundle on the performance of cisplatin-loaded micelles.
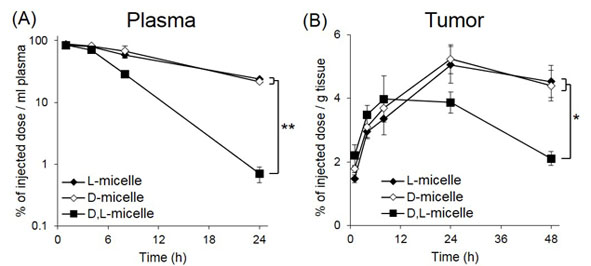
Fig. 1. (A) Plasma retention and (B) tumor accumulation of the series of micelles after i.v. administration. Data are means±S.E., n = 5, *P < 0.01, **P < 0.001.
Conclusion: Introducing higher-order structures can be a novel and effective strategy to control the drug delivery performance of polymeric micelles without losing functionality of the constituting polymer chains.
References:
[1] Cabral et al., J. Control. Release 190 (2014) 465
[2] Matsumura et al., Cancer Res. 46 (1986) 6387
[3] Mochida et al., ACS Nano 8 (2014) 6724