Introduction: As quality of life improves there is an increase in the use of implantable biomaterials as medical devices. Although biomaterials are designed to be inert they can trigger host inflammation at the interface, which left untreated can lead to device failure. α-Melanocyte stimulating hormone (α-MSH) is a pleiotropic peptide hormone recognised for its anti-inflammatory properties. Our initial work successfully immobilised a synthetic version of α-MSH [GKP(D)V] onto glass using calixarene surface chemistry which attenuated an inflammatory response in vitro[1]. Our latest work incorporates α-MSH onto aryl azide functionalised silanes offering micropatterning capabilities via photolithography based on an established method within the group[2]. The present aim of this work is to optimise the photochemistry of the aryl azide silanes by immobilising a variety of bioactive molecules to provide a direct method for the surface modification of biomaterials. As well as observing the effects of surface patterning we aim to create highly ordered coatings with multifunctional bioactive properties.
Materials and Methods: Glass coverslips were surface functionalised with calixarene-PEG-3-GKP(D)V and subjected to biological inflammatory testing (Fig.1). Thereafter, glass coverslips were coated with a photoactive aryl azide organosilane and the surface irradiated to immobilise a modified azido-PEG-6-GKP(D)V tether for micropatterning and subjected to inflammatory testing.
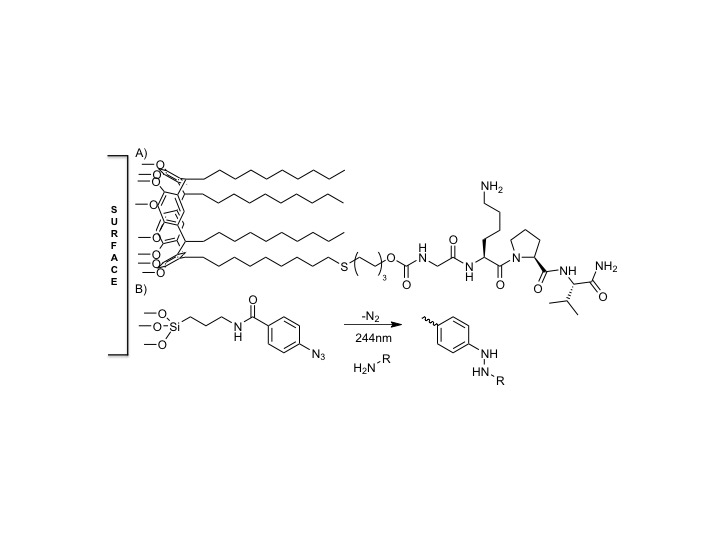
Figure 1: A) Synthetic α-MSH (GKP(D)V) attached to a calixarene via PEG-3 tether for surface modification. B) Summary of surface patterning on aryl azide silane surfaces via photolithography[2].
Surfaces were characterised by XPS, SIMS, ellipsometry, and contact angle goniometry. Human fibroblasts, Schwann cells, and NIH-3T3 fibroblasts were seeded onto surface treated coverslips before stimulation with TNF-α or LPS. To monitor inflammatory cell-signalling activity, the p65 subunit of NF-κB was immunolabeled and quantified by confocal microscopy.
Results and Discussion: XPS and SIMS confirmed that GKPV(D) was immobilised onto calixarene and aryl azide coated surfaces. Calixarene-PEG-GKP(D)V surface coatings inhibited TNF-α stimulated NF-κB activity by 23% ± 4% (n=3, p=0.001). Inhibition was comparable to control untreated surfaces stimulated with TNF-α and 10-9M GKP(D)V (39% ± 3%; n=3, p=0.003) indicating that coatings require relatively a low peptide load[1]. Similarly, the aryl azide silanated surfaces with N3-HEG-GKP(D)V also reduced NF-κB activity by 28% ± 4% (n=3, p=0.01).
Conclusions: Data demonstrates that both calixarene and silane immobilisation methods can inhibit NF-κB cell signalling activity involved in the inflammatory response. Current work is focussed on how surface patterning controls anti-inflammatory properties and extends through to carbon monoxide releasing molecules using inorganic carbonyl compounds as a therapeutic approach.
Engineering and Physical Sciences Research Council (EPSRC), UK
References:
[1] M. Charnley, K. Faifull-Smith, N.W. Williams, J.W. Haycock, Advanced Materials, 21, 2009, 2909-2915.
[2] O. El Zubir, I. Barlow, N.W. Williams, G.J. Leggett, Langmuir, 9, 2013, 1083-1092.