Introduction: Chemoresistance is a big barrier for tumor chemotherapy although the chemotherapy is one of the important tumor therapies. Chemoresistance increases in the cells at higher malignancy. Genetic mutations have been focused to understand the mechanisms of tumor chemoresistance. Recently, it has been also pointed out that extracellular microenvironment including extracellular matrix (ECM) is one of the factors to determine the chemoresistance. ECM is dynamically remodeled during tumor progression. However, it is not clear how ECM and its remodeling influence tumor cell chemoresistance. Here, I tried to reconstruct in vitro ECM models mimicking ECM in tumor tissue at each malignancy using in vitro tumor cell culture and decellularization technique. These ECM models were termed as “staged tumorigenesis-mimicking matrices”. I examined the chemoresistance and its expression mechanism of colorectal tumor cells on staged colorectal tumorigenesis-mimicking matrices to study the role of ECM in chemoresistance expression comprehensively.
Materials and Methods: Colorectal tumor cell lines, HT-29 (metastatic), SW480 (non-metastatic), and CCD-841-CoN (benign) were cultured for 2 weeks on tissue culture plates to deposit ECM proteins secreted from the cells. After the culture, the cells were specifically removed from the culture with the treatments of Triton X-100 and NH4OH, followed by DNase and RNase treatments. Fresh tumor cell lines were seeded on the obtained ECM and were exposed 5-fluorouracil (5-FU) for 3 days. The grown cell number was examined by WST-8 assay. Akt and Extracellular signal-Regulated Kinases (ERKs) phosphorylation levels were compared by Western blot analysis. The expression levels of 5-FU resistance-relating genes were measured by real-time PCR analysis.
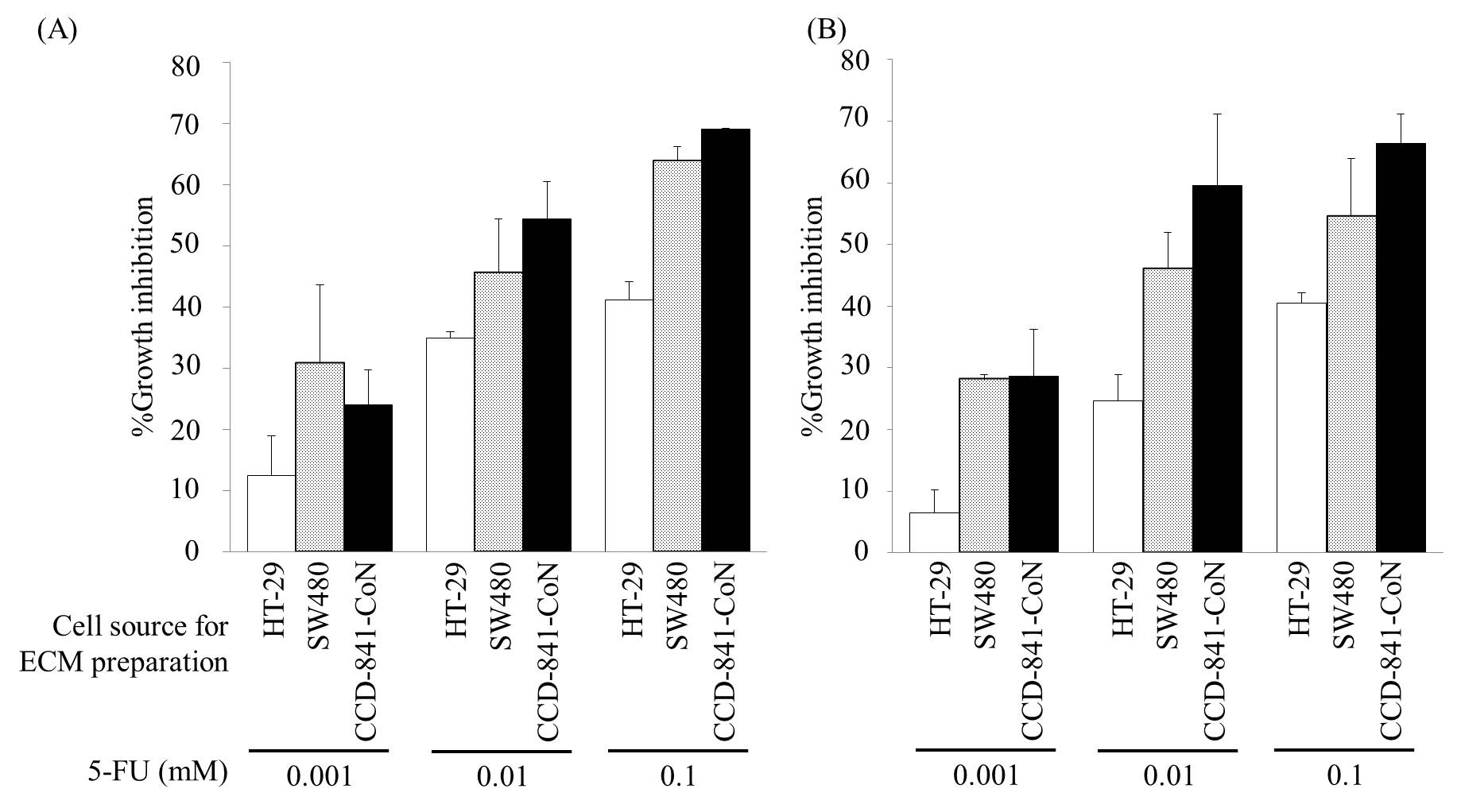
Results and discussion: The resistance of HT-29 and SW480 against 5-FU increased on only HT-29-derived ECM, indicating that the chemoresistance increased on ECM at higher malignancy (Figure 1A and 1B).
Akt phosphorylation level in HT-29 was higher on HT-29-derived ECM than the other ECM, suggesting that Akt partially contributed to the chemoresistance on HT-29-derived ECM (Figure 2A).
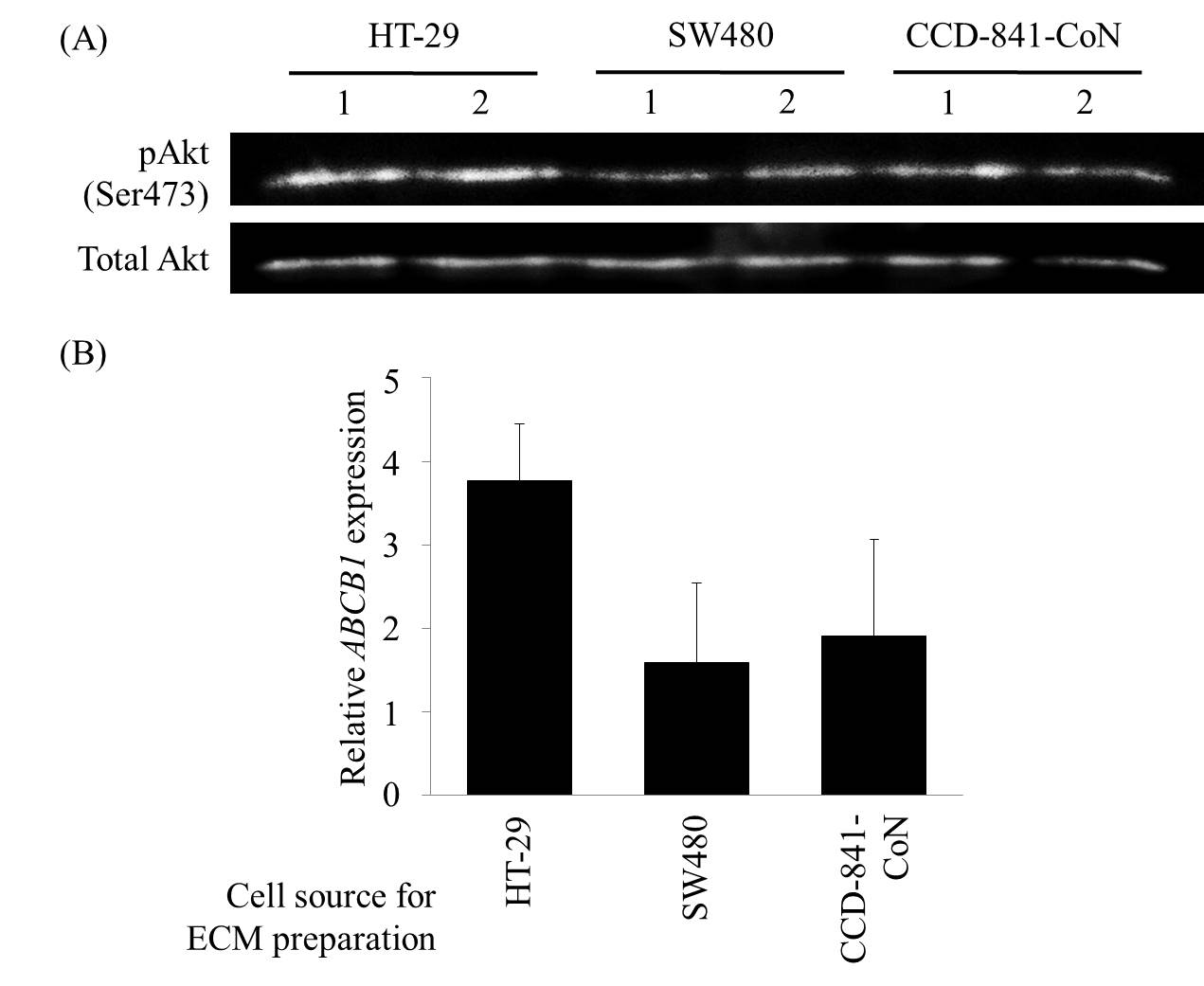
ERK phosphorylation levels were similar among staged colorectal tumorigenesis-mimicking matrices. The expression levels of the transporters to exclude 5-FU, ABCB1 and ABCC1, increased in HT-29 on HT-29-derived ECM, suggesting that higher ABCB1 and ABCC1 expression increased 5-FU resistance on HT-29-derived ECM (Figure 2B).
Conclusion: ECM remodeling during the colorectal tumor progression increases the chemoresistance through the activation of Akt and the increase of drug exclusive transporters expression. Staged tumorigenesis-mimicking matrices are useful in vitro ECM models for the comprehensive study of ECM roles in tumor biology including the exhibition of chemoresistance.