Introduction: During the last decade, tremendous efforts have been attained in the development of stimuli-responsive polymeric nanocarriers for the controlled delivery of various anti-cancerous drugs[1]. Physiological stimuli-responsive nanocarriers provide the feasible platforms for cancer therapy due to their improved anticancer efficiency as well as reduced systematic cytotoxicity via the tumor specific enhanced permeability and retention (EPR) effect[2]. It is interesting to note the pH-sensitive surface charge-conversion strategy, which is potential for enhancing tumor cell internalization efficiency. In this study, we attempt to engineer this smart tumor-acidity activated surface charge-conversion function into a biodegradable amphiphilic block copolymers for anticancer application.
Materials and Methods: Poly(D, L-lactide)-block-poly(2-aminoethyl methacrylate) (PLA-b-PAEMA, MW 8900, PDI 1,24) was firstly synthesized through ring-opening polymerization (ROP) and subsequently atom transition radical polymerization method. PLA-b-PAEMA/DMMA was obtained by using 2,3-dimethykmaleic anhydride (DMMA) modified PLA-b-PAEMA.
Results and Discussion: The well-defined PLA-b-PAEMA is further modified by DMMA and succinic anhydride (SA) to yield the tumor-acidity activated surface charge-conversional block copolymer (PLA-b-PAEMA/DMMA) and the control sample (PLA-b-PAEMA/SA). In this design, the biodegradable PLA block and the functional PAEMA/DMMA (or PAEMA/SA) block provide the amphiphilic driving force to self-assemble into nanocarriers, which have PLA aggregated hydrophobic inner core and PAEMA/DMMA (or PAEMA/SA) outer corona, in aqueous solution. As compared with the invariable negative surface charge of PAEMA/SA block, PAEMA/DMMA block shows the tumor-acidity activated surface charge-conversion feature, i.e., exhibiting negatively charged surface during the blood circulation time and positively charged surface in response to slight tumor acidic environment through the hydrolysis of DMMA functional agents. Thus, after the loading of positively charged doxorubicin hydrochloride (DOX·HCl) into the negatively charged corona structure through electrostatic attraction, the DOX·HCl-loaded PLA-b-PAEMA/DMMA carrier (PLA-b-PAEMA/DMMA@DOX·HCl) is expected to prolong blood circulation time because its “stealthy” negatively charged surface could effectively decrease the undesired consumption by the reticuloendothelial system (RES) and the protein adsorption in vivo, and promote the anti-cancer therapeutic efficiency though the tumor activated positively charged surface for enhanced tumor cell adhesion and targeted drug release (Scheme 1).
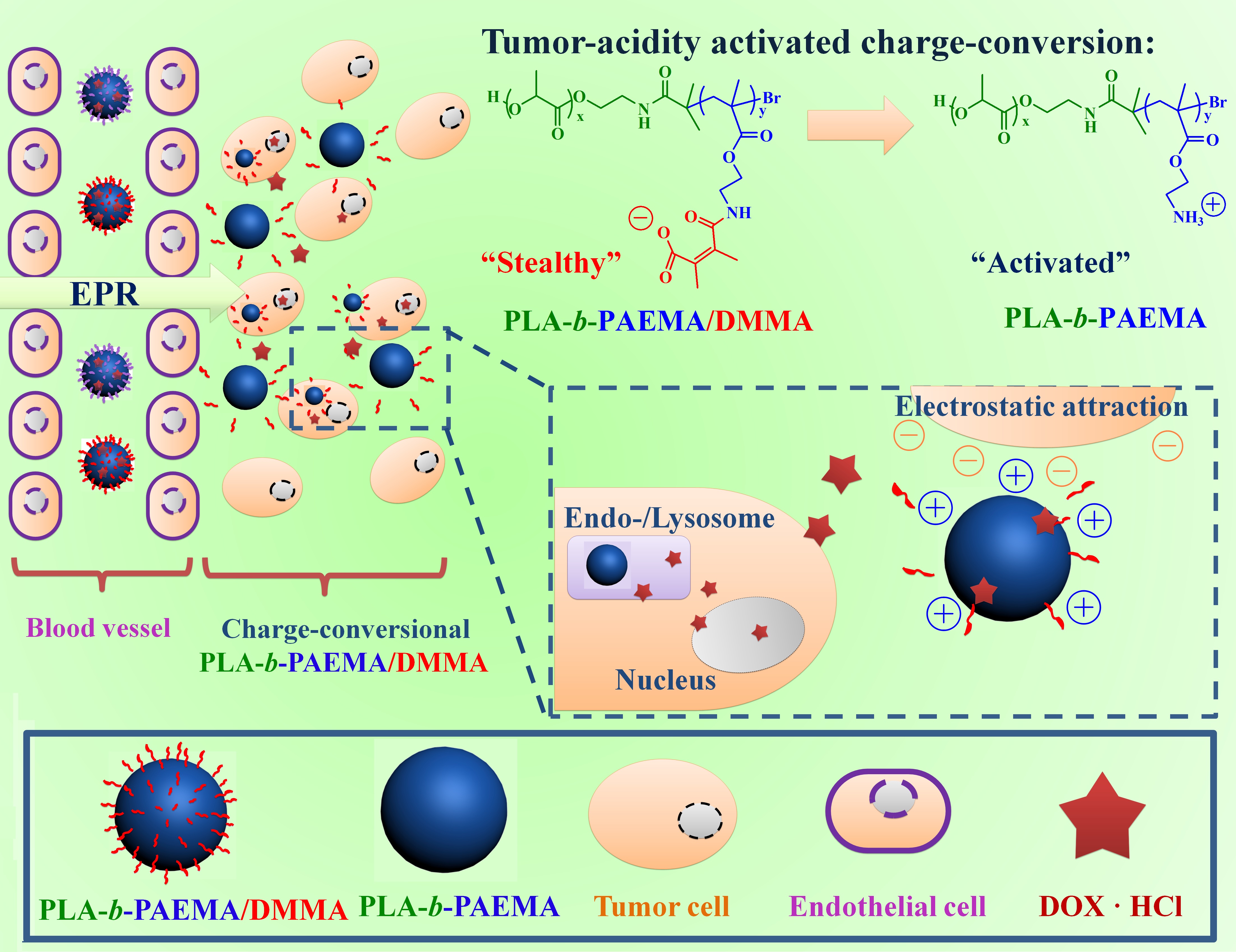
Scheme1. Illustration of tumor-acidity activated carriers for enhanced cell uptake and targeted drug release.
Conclusions: In summary, we construct a smart nanocarrier based on biodegradable PLA-b-PAEMA/DMMA copolymer, which could convert surface charge from negative to positive in response to the tumor-acidity. It exhibits various superiorities for tumor-specific drug delivery, including long blood circulation time, enhanced tumor-cell adhesion, tumor targeted drug release and additional cytotoxicity for tumor cells after the charge-conversion. Thus, this biodegradable smart carrier integrated high drug delivery efficiency and well-improved biocompatibility provides a feasible platform for the ideal anti-tumor therapy.
Financial support from the National Natural Science Foundation of China (51322303); Financial support from the Foundations of Sichuan Province (2012JQ0009)
References:
[1] Mura S., Nicolas J., Couvreur P., stimuli-responsive nanocarriers for drug delivery [J], Nat. Mater., 2013(12): 991-1003.
[2] Wu W., Wang J., Lin Z., tumor-acidity activated surface charge-conversion of polymeric nanocarriers for enhanced cell adhesion and targeted drug release [J], Macromol. Rapid Commun., 2014, 35 (19): 1679-1684.