Introduction: Many studies have demonstrated that physical nanotopography and chemical cues of scaffolds exert important influence on cell behavior. Electrospun aligned nanofibers have been used to mimic the smooth muscle layer of blood vessels[1],[2], however, engineering the aligned ultrafine fiber surface with nanotopographical and biological cues to examine their impact on cell responses have not been paid due attention.
Materials and Methods: Highly aligned ultrafine fibers of poly(L-lactic acid) (PLLA) with different surface features, i.e., nano-pore or hyaluronan (HA) coating, were prepared via an established stable jet electrospinning (SJES) method[3],[4]. Specifically, ellipse-shaped nano-pores were generated on the fiber surfaces by varying ambient humidity (45~75%), whereas the HA-functionalized PLLA nanofibers in core-shell structure (denoted as HA/PLLA) were prepared by employing a coaxial spinneret in the SJES. To stabilize the HA-coating layer, the HA/PLLA nanofibers were subsequently subjected to cross-linking[5]. The two types of well-aligned PLLA fibers were thoroughly characterized by different techniques. Thereafter, human umbilical arterial SMCs (HUASMCs) were seeded respectively onto these aligned fibers to examine cellular responses including cell adhesion, proliferation, morphology changes (e.g., elongation and orientation), synthesis of vascular matrix proteins and phenotypic expression in vitro. Finally, vascular conduits (∅i.d. = 3 mm) made of circumferentially aligned HA/PLLA nanofibers were constructed for implantation in rabbit carotid artery for vascular regeneration in vivo.
Results and Discussion: Previoiusly, we have demonstrated that presence of nano-pores gave rise to favorable cellular responses, leading to enhanced cell attachment, proliferation, alignment, synthesis of the vascular matrix proteins (e.g., collagen), and up-regulation of desired contractile phenotype marker α-SMA and down-regulation of the synthetic phenotype marker OPN [6].
In current study, highly aligned HA/PLLA nanofibers (~800 nm) in core-shell structure were readily fabricated and characterized by SEM, TEM and FTIR, respectively (Fig.1A-C). In biological tests in vitro, the aligned HA/PLLA nanofibers were found to generally support HUASMCs to elongate, orientate, and proliferate along the fiber axis remarkably (Fig.1D-F). There appeared also significantly up-regulated contractile protein (Fig.1G) and gene (Fig.1H-I) expressions in the HUASMCs cultured on the core-shell nanofibers after 5 days of culture. Animal test results show that the tubular graft made of aligned HA/PLLA nanofibers was covered with identifiable capillary vessels after 6 weeks of implantation (Fig.1J). Moreover, simultaneous regeneration of circumferentially oriented vSMCs in contractile phenotype and luminal endothelium was observed (Fig.1K-L), demonstrating the strong promoting role of HA-coating layer (data relating to the control of PLLA conduits are not shown). As the implanted HA/PLLA grafts were cell-free, regeneration of native-like neoartery in 6 weeks suggests their potent capability of recruiting host cells and adaptivity to the native environment by coordinating with the host self-remodeling.
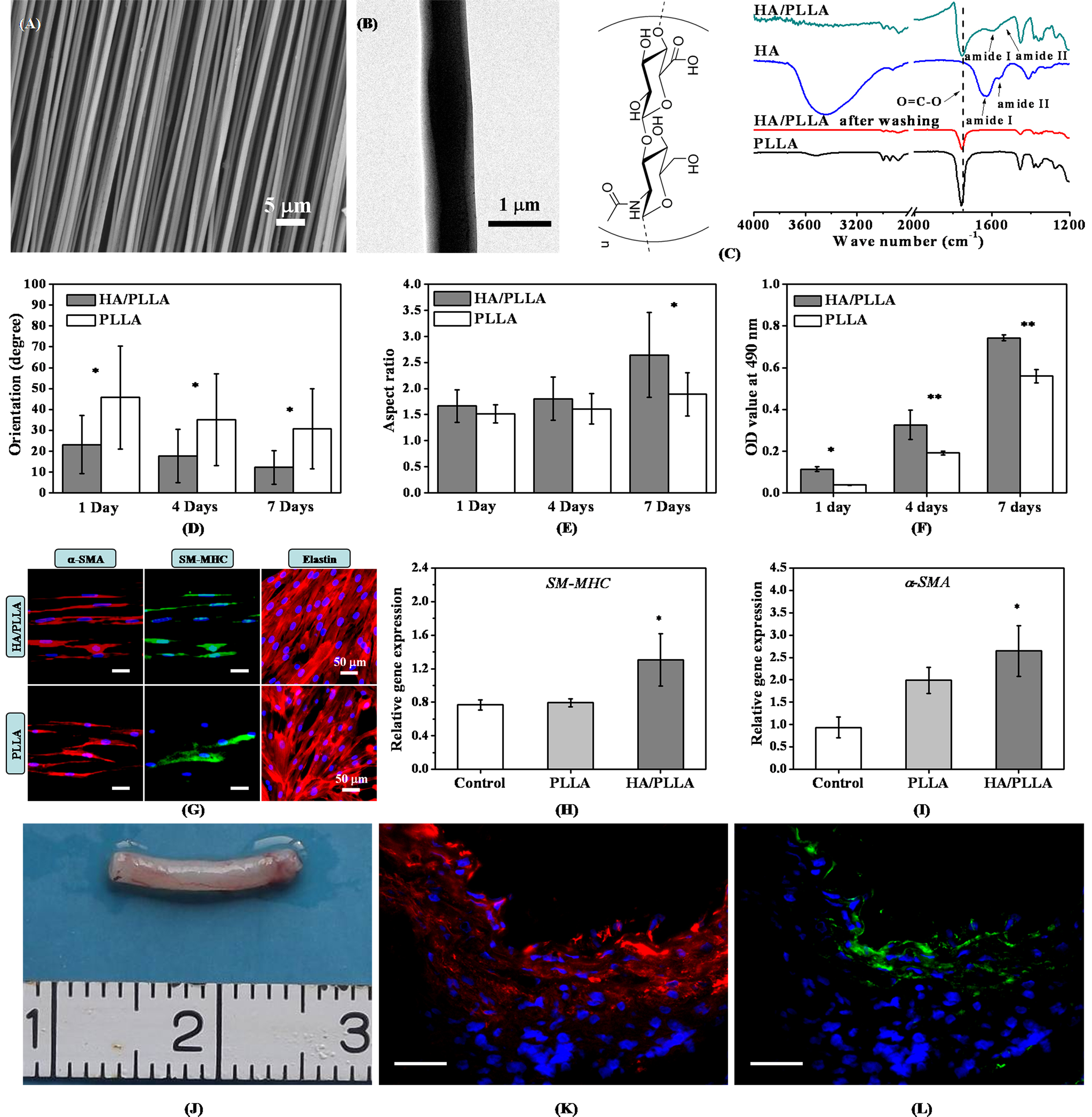
Fig.1 Fabrication, characterization and biological evaluations of the highly aligned HA/PLLA nanofibers on promoting vSMCs' responses. Scar bar in (K, L) is 100 µm. *p<0.05, **p<0.01.
Conclusions: This work demonstrated that engineering surfaces of the highly aligned electrospun PLLA fibers with nanotopographical feature or HA-coating layer may be used as effective means for enhancing the responses of vSMCs toward development into the desired contractile phenotype.
This work was partially supported by the National Natural Science Foundation of China (51073032, 31570969), the Natural Science Foundation of Shanghai (15ZR1400500), the Key Basic Research Foundation of Shanghai Committee of Science and Technology (14JC1490100), and the Fundamental Research Funds for the Central Universities by the Ministry of Education of China (2232013D3-13, 15D110538).
References:
[1] Wang Y, Shi H, Qiao J, et al. Electrospun tubular scaffold with circumferentially aligned nanofibers for regulating smooth muscle cell growth. ACS Applied Materials &Interfaces, 2014, 6 (4):2965-2969.
[2] Nivison-Smith L, Weiss A S. Alignment of human vascular smooth muscle cells on parallel electrospun synthetic elastin fibers. Journal of Biomedical Materials Research Part A, 2012, 100A (1):155-161.
[3] Yuan H H, Zhao S F, Tu H B, et al. Stable jet electrospinning for easy fabrication of aligned ultrafine fibers. Journal of Materials Chemistry, 2012, 22 (37):19634-19638.
[4] Zhou Q H, Bao M, Yuan HH, et al. Implication of stable jet length in electrospinning for collecting well-aligned ultrafine PLLA fibers. Polymer, 2013, 54 (25): 6867-6876.
[5] Tomihata K, Ikada Y. Crosslinking of hyaluronic acid with glutaraldehyde. Journal of Polymer Science Part A-Polymer Chemistry, 1997, 35 (16): 3553-3559.
[6] Zhou Q H, Xie J, Bao M, et al. Engineering aligned electrospun PLLA microfibers with nano-porous surface nanotopography for modulating the responses of vascular smooth muscle cells. Journal of Materials Chemistry B, 2015, 3 (21): 4439-4450.