Development of a biomimetic nanocomposite gradient scaffold to mimic the natural osteochondral structure for tissue repair
-
1
King's College London, Tissue Engineering & Biophotonics Division, United Kingdom
-
2
University of Salento, Department of Engineering for Innovation, Italy
Introduction: The natural osteochondral (OC) tissue exhibits a unique gradient structure which varies with distance from the articular cartilage to subchondral bone[1]. In this study, a biomimetic collagen-based scaffold reinforced with nanohydroxyapatite (nHAp) has been developed to obtain an engineered device which mimics the gradient structure of the natural tissue for OC defects repair[1],[2]. Scaffolds were analysed to verify chemical and phase compositions, porosity architecture and mechanical properties. Furthermore, they were seeded with primary human mesenchymal stem cells (hMSCs) and examined for differentiation towards osteo- and chondrogenic lineages[3].
Materials and Methods: Bovine collagen I (Coll) was used as a collagen source. Hydrated CaO from hen eggshell and H3PO4 were used for nHAp synthesis. nHAp was directly nucleated into collagen suspensions and highly porous scaffolds were obtained using a one-step freeze-drying process. FTIR, XRD, SEM, EDS and micro-CT analyses were carried out to check chemical and phase compositions and pores architecture of the scaffold. Mechanical properties were verified by compression and tension tests in wet state. Primary hMSCs were seeded on scaffolds and then conditioned to induce terminal differentiation in osteo- or chondrogenic conditions. Cell proliferation (alamarBlue) and viability (Live&Dead) were verified up to 28 days. The morphology of cells on the scaffolds was evaluated using SEM and fluorescence microscopy. qPCR was used to verify the expression of specific genes for osteo- (ON, RUNX2) and chondrogenic (SOX9, COL2A1) differentiation.
Results and Discussion: Physicochemical analyses confirmed that scaffolds were composed of collagen and nHAp, and both materials were chemically integrated with each other. In particular, SEM, EDS and micro-CT showed the gradient distribution of mineral phase and interconnected porosity throughout the scaffold.
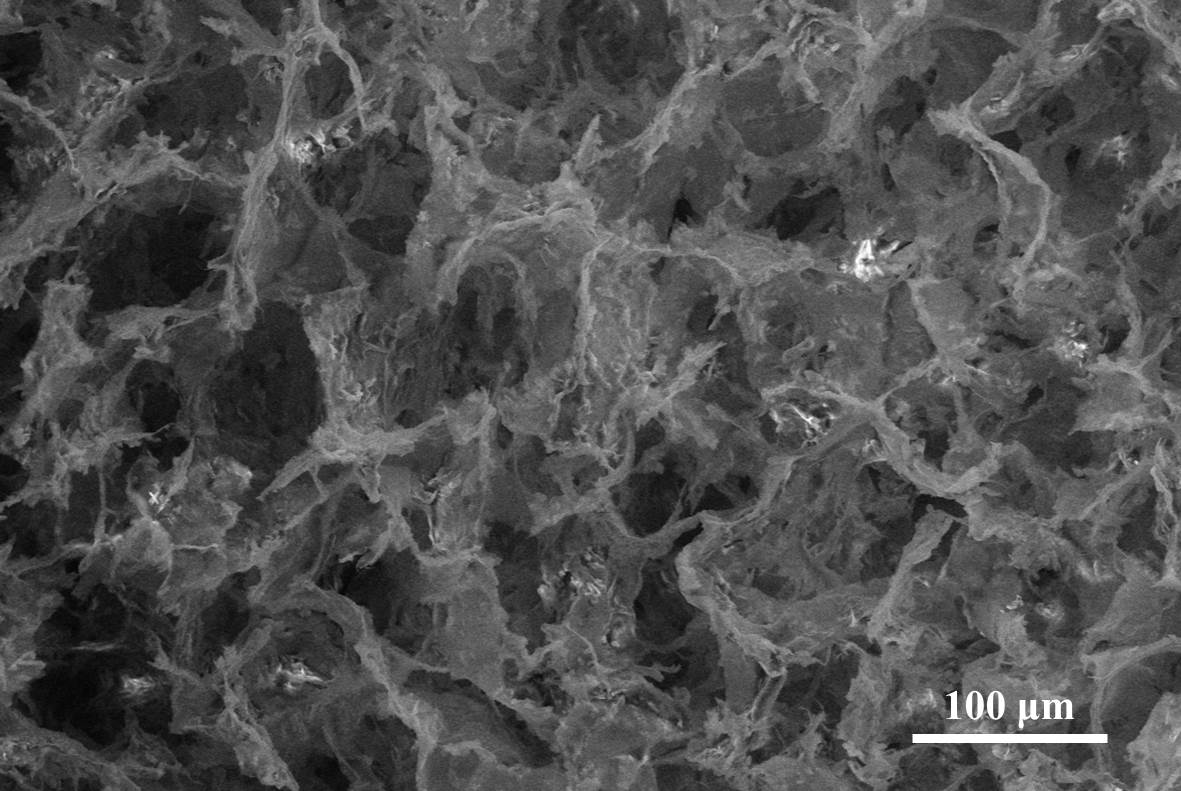
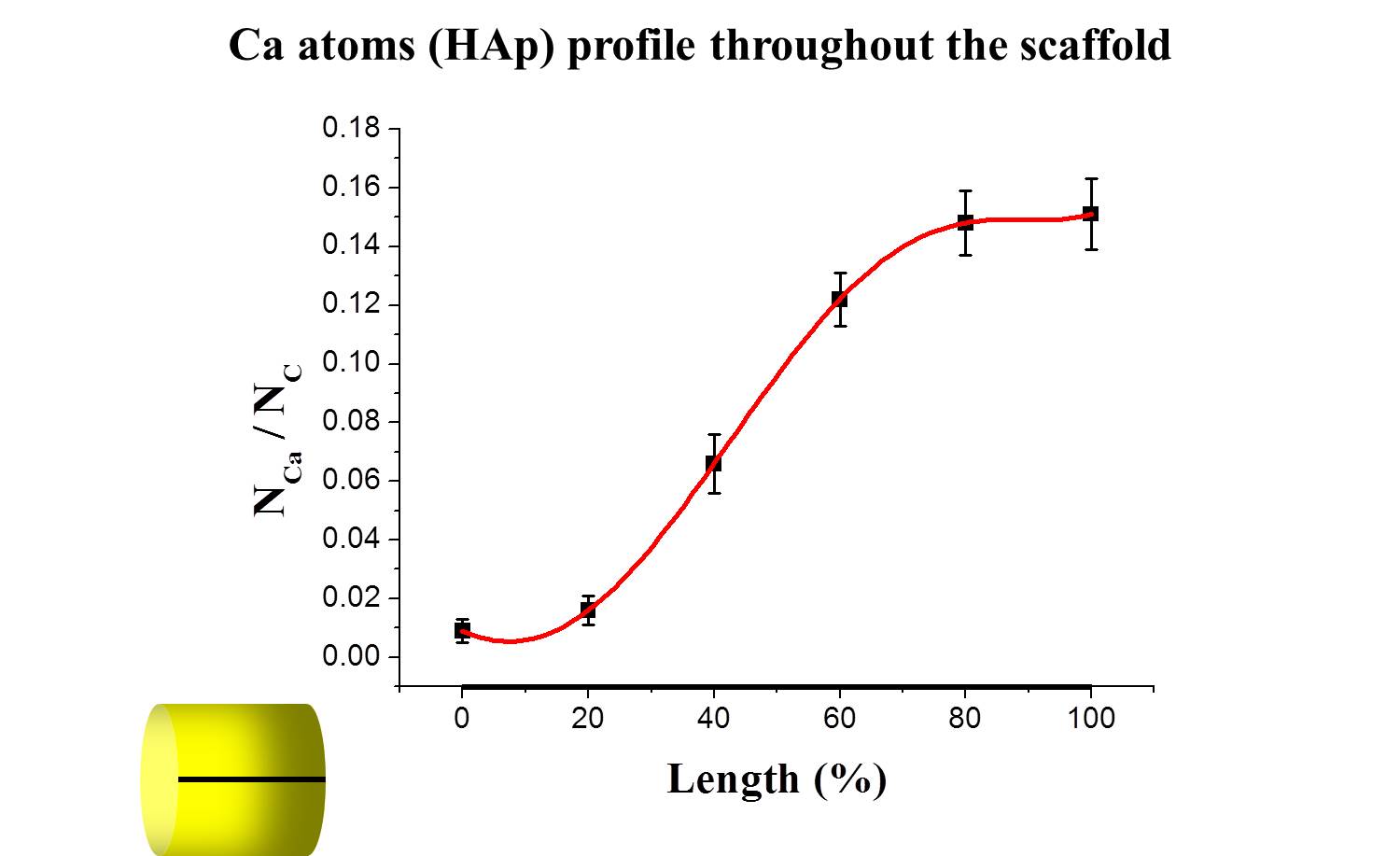
Compression and tension tests confirmed the mechanical stability of the device and showed improved mechanical properties with the increase of the nHAp content. The gradient scaffold exhibited excellent biocompatibility and allowed cell migration with higher cell proliferation and viability compared to non-gradient nHAp/Coll scaffolds. Cells on the gradient scaffold demonstrated different morphologies after 28 days in osteo- or chondrogenic condition. This was confirmed by qPCR analysis where specific genes (ON, RUNX2, SOX9, COL2A1) showed an upregulation compared to non-gradient scaffolds when kept in osteo- or chondrogenic condition.
Conclusion: The biomimetic scaffold was successfully fabricated with a gradient distribution of mineral phase and interconnected porosity throughout the scaffold as desired. The scaffold showed excellent mechanical and biological properties allowing stem cells proliferation and differentiation in osteo- and chondrogenic condition. On the basis of these excellent results, in vivo studies on an animal model (sheep) are currently ongoing.
References:
[1] Nukavarapu S. et al., Biotechnology Advances. 31:706-721, 2013
[2] Zhang T. et al., Biomaterials. 38:72-85, 2015
[3] Lam J. et al., Acta Biomaterialia. 10:1112-1123, 2014
Keywords:
Biomimetic,
Scaffold,
composite,
Tissue Regeneration
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomimetic materials
Citation:
Parisi
C,
Salvatore
L,
Gurav
N,
Madaghiele
M,
Di Silvio
L and
Sannino
A
(2016). Development of a biomimetic nanocomposite gradient scaffold to mimic the natural osteochondral structure for tissue repair.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01161
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.