Introduction: Aortic stenosis and regurgitation are the most common aortic diseases leading to valve failure in Europe and North America. The aim of this study was to develop a TEHV based on elastin-like recombinamers (ELRs). ELRs are bioengineered polymers based on the natural elastin, produced by recombinant techniques[1] and positioned midway between natural products and synthetic polymers, due to they keep the biological origin and nature in addition to the advantages of an engineered material[2], for instance, the exhaustive control over their composition, functionality and tunable mechanical properties. They possess an extremely low polydispersity, thermosensitive behavior, a very low thombogenicity[2] and the possibility to include different bioactive sequences for cell adhesion and proliferation or sequences sensitive to enzymes[1].
Materials and Methods: The ELRs used in this work were obtained by using standard genetic engineering techniques. Their purification was based on the intrinsic thermal behavior of these compounds[3]. ELRs were chemically modified by transformation of the α-amine group in the lateral lysine chain to bear cyclooctine and azide groups. Valves were formed by catalyst free click reactions between an azide group and an activated cyclooctine group[4]. Solutions of ELRs-cyclooctine and ELRs-azide (90 mg/mL) were prepared in PBS at the desired concentration and kept at 4°C for at least 24h. 30 million cells were suspended in 500 µL of PBS and mixed with the HRGD6-N3 solution previously prepared. 1.5 mL of each polymer solution, total volume of 3 ml) (VKVx24-cyclo and HRGD6-N3 (final concentration 75 mg/mL) containing the cells were co-injected inside a 15 mm diameter aortic shape mould and kept 45 minutes at room temperature. Then the valves (n=3) were cultivated under static conditions for 7 days following dynamic conditioning for 14 days at 37 °C, 5% CO2 and 21% O2 in a custom-made bioreactor[5].
Results and Discussion: The valves were fully functional with an unobstructed opening (systolic phase) and complete closure (diastolic phase), as verified by frames extracted from high-speed movies and ultrasound.
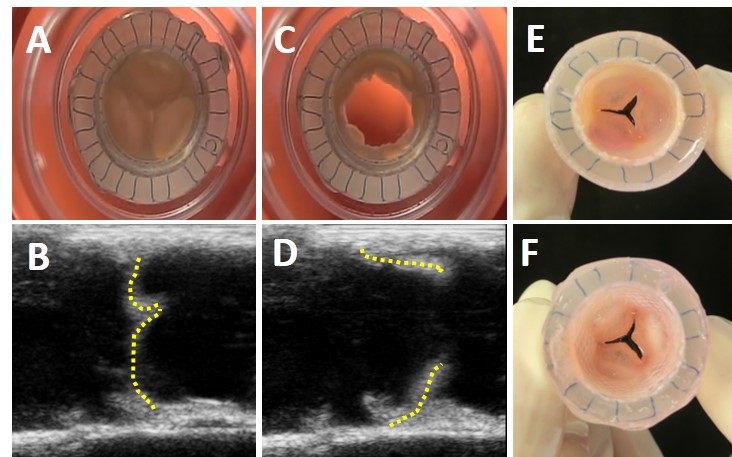
Figure 1. Valve`s functionality and macroscopic appearance after 21 days of cultivation. Frames showing the closed (A) and open (B) positions of the valve, (B and D) ultrasound echography pictures of the EA-valves in the closed and open position respectively (dashed line was added to clarify the ultrasound images), and macroscopic appearance of the valve from the vascular side (E) and ventricular side including the wall (F).
Immunohistochemistry revealed a pronounced deposition of collagens type I and III and alpha-SMA active cells aligned within the longitudinal direction of both wall and leaflets.
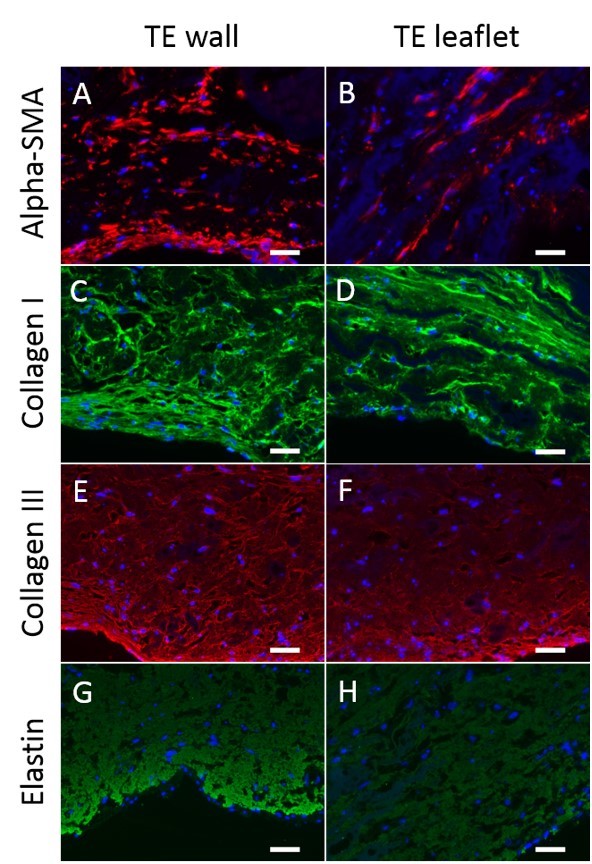
Figure 2. Tissue analysis of the EA-valves after 21 days of in vitro culture. Immunohistochemical staining against alpha-smooth muscle actin (A, B), collagen I (C, D), collagen III (E, F) and elastin (G, H). DAPI was used for nuclei staining in all samples studied. Scale bars 50 mm.
The applied conditioning protocol resulted in an increase of the mechanical properties as determined by burst strength measurements. After conditioning, wall and leaflet tissues burst at 579.5 ± 145.8 mmHg and 642.8 ± 135.5 mmHg respectively. Collagen content of the walls and leaflets reached 22.6 ± 3.3 % and 30.7 ± 2.4 % respectively, relative to the native human aortic valve.
Conclussions: The previous results show the first TEHV made exclusively of ELRs as scaffold. These valves exhibited an excellent mechanical properties, good cell proliferation and development of new extracellular matrix, as immunochemistry and burst strength test reveals. These results highlight the potential of these functional TEHVs to be implanted in the aortic position.
European regional development fund (ERDF), from the MINECO (MAT-2010-15982, MAT2010-15310, PRI-PIBAR-2011-1403 and MAT2012-38043), the JCyL (projects VA049A11, VA152A12 and VA155A12), the CIBER-BBN, and the JCyL and the Instituto de Salud Carlos III under the “Network Center of Regenerative Medicine and Cellular Therapy of Castilla and Leon”; People Programme (Marie Curie Actions) of the European Union´s Seventh Framework Programme FP7/2007-2013/ under REA grant agreement n°317512; Funding was also provided by the People Programme (Marie Curie Actions) of the European Union´s Seventh Framework Programme FP7/2007-2013/ under REA grant agreement n°317512, by the Integrated Interdisciplinary Institute of Technology for Medicine (I³TM) of RWTH Aachen University [Seed-Fund SF_14-4-08] and by the the START-Program of the Medical Faculty of RWTH Aachen University [Innenfond 691348].; “The THEGRAIL project is funded by the European Union’s ‘Seventh Framework’ Programme for research, technological development and demonstration under Grant Agreement n° HEALTH.2011.1.4-2- 278557 From 2012-01-01 to 2016-12-31″
References:
[1] Rodríguez-Cabello JC, Martín L, Alonso M, Arias FJ, Testera AM. “Recombinamers” as advanced materials for the post-oil age. Polymer 2009;50:5159-69.
[2] Gonzalez de Torre I, Wolf F, Santos M, Rongen L, Alonso M, Jockenhoevel S, et al. Elastin-like recombinamer-covered stents: Towards a fully biocompatible and non-thrombogenic device for cardiovascular diseases. Acta Biomater 2015;12:146-55.
[3] Girotti A, Reguera J, Rodriguez-Cabello JC, Arias FJ, Alonso M, Matestera A. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater Med 2004;15:479-84
[4] Gonzalez de Torre I, Santos M, Quintanilla L, Testera A, Alonso M, Rodriguez Cabello JC. Elastin-like recombinamer catalyst-free click gels: characterization of poroelastic and intrinsic viscoelastic properties. Acta Biomater 2014;10:2495-505
[5] Weber M, Heta E, Moreira R, Gesche VN, Schermer T, Frese J, et al. Tissue-engineered fibrin-based heart valve with a tubular leaflet design. Tissue engineering Part C, Methods 2014;20:265-75.