Introduction: A modular bone tissue engineering (TE) approach that builds larger, implantable constructs from small cell laden modules could circumvent some pitfalls of traditional bone TE (limited cell migration, inadequate scaffold mineralization, etc.). One modular TE strategy relies on composites of natural polyelectrolytes, such as glycosaminoglycans (GAGs) and chitosan, to construct modular units. Chondroitin 4-sulfate (C4S), the primary GAG in bone, facilitates hydroxyapatite (HAP) nucleation in vitro [1]. In addition, exogenous HAP can stimulate osteogenesis of mesenchymal stem cells (MSCs) [2]. The modular TE design described here involves the encapsulation of mesenchymal stem cells (MSCs) and HAP in a polyelectrolyte membrane composed of C4S and chitosan (Ct) to create micromodular units capable of being fused into larger implantable constructs. The influence of the extent and location of mineral deposition within the micromodules on fused construct mechanical properties is presented here.
Methods: HAP and MSCs were encapsulated in spherical C4S/ct micromodules by polyelectrolyte complexation [3]. Four conditions were investigated: micromodules formed without exogenous HAP cultured in standard medium (E) or osteogenic medium (O) for four weeks, and similar micromodules containing HAP microgranules (cultured in standard, EH or osteogenic, OH medium). After 4 weeks of culture, micromodules were fused by the deposition of additional C4S/chitosan polyelectrolyte complex between the micromodules, to create larger fused constructs. The extent and organization of mineral deposition within micromodules/constructs was determined by μCT analysis after 4 weeks of static culture. Yield stresses under uniaxial compression were determined for each construct.
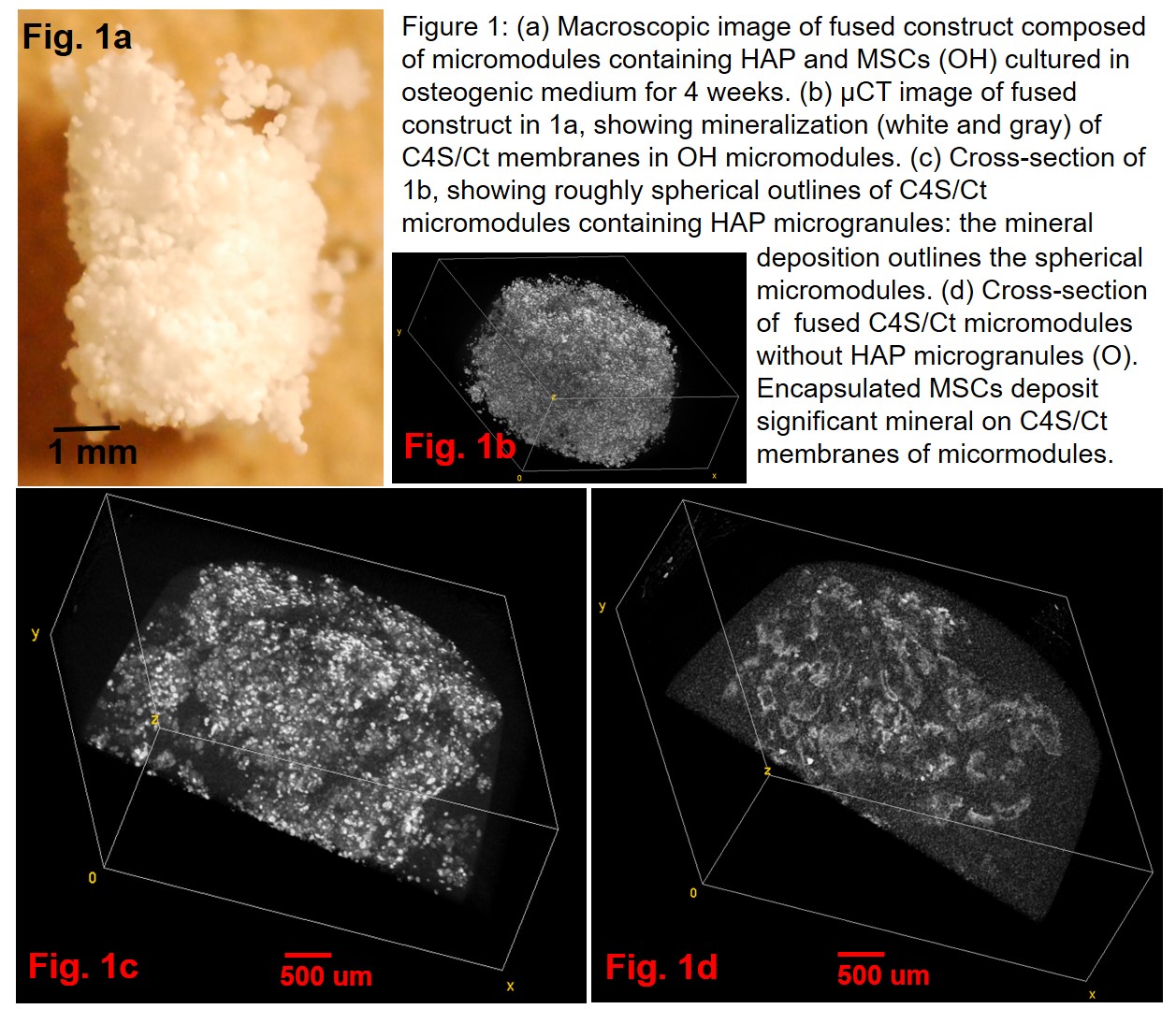
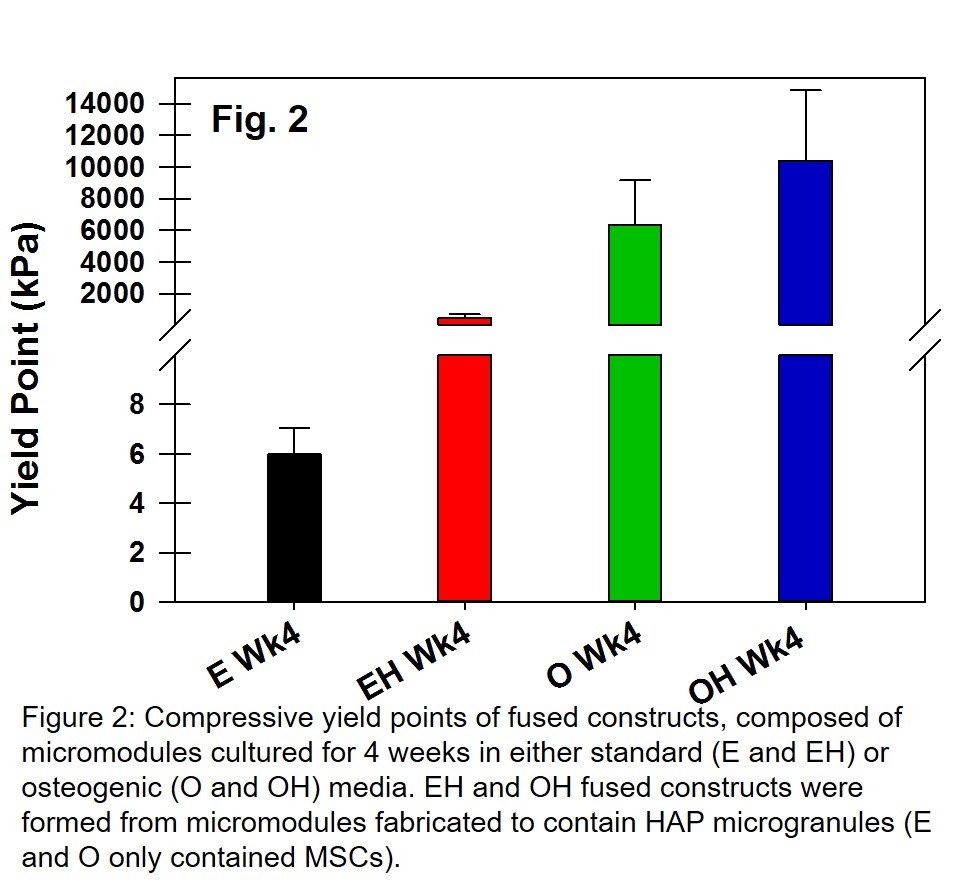
Results and Discussion: Complexation between C4S and chitosan was found to adequately fuse mineralized micromodules containing differentiating MSCs (Fig. 1a and 1b). μCT analysis demonstrated that significant mineral deposition occured on the C4S/chitosan membrane enveloping the HAP and osteogenic MSCs (Fig. 1c). Moreover, fused constructs fabricated from micromodules containing osteogenic MSCs (O and OH micromodules) exhibited significantly higher compressive yield points (Fig. 2), compared to micromodules containing standard cultured MSCs (EH and E). Including HAP in the micromodules significantly enhanced the mechanical properties of tissue constructs created from fused micromodules. Interestingly, the location of mineralization was the primary determinant of mechanical properties: constructs composed of actively mineralized micromodules without HAP (O) had significantly higher yield stresses than the non-mineralized constructs containing HAP (EH), possibly due to the localization of mineral deposition to the micromodule C4S/Ct membranes (Fig. 1d). This data strongly suggests that mineralization reinforces the C4S/chitosan membranes, so that compressive stresses are effectively dissipated across the micromodules, resulting in fused constructs with higher compressive strength.
Conclusion: The mechanical properties demonstrate that the in vitro mineralization of the C4S/chitosan module membrane is the primary determinant of mechanical properties in the fused constructs. Results show this micromodular unit design can form the basis of a modular TE solution to regenerate mechanically robust bone.
References:
[1] Slater, M., J. Patava, and R.S. Mason, Role of chondroitin sulfate glycosaminoglycans in mineralizing osteoblast-like cells: effects of hormonal manipulation. J Bone Miner Res, 1994. 9(2): p. 161-9.
[2] Peng, H., et al., Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology, 2012. 23(48): p. 485102.
[3] Tiruvannamalai-Annamalai, R., D.R. Armant, and H.W. Matthew, A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PLoS One, 2014. 9(1): p. e84287.