Introduction: Cisplatin (cis-diamminedichloroplatinum) is one of the widely used anticancer agents to treat a variety of tumours[1]. However, due to dose limiting toxicities and side effects such as gastrointestinal disturbance, its therapeutic effect on many applications is restricted[2]. Recently, many types of controlled release formulations have been designed by several researchers to enhance the drug dosage in tumour site and avoid its toxicity levels caused by administration via conventional methods. The restrictions in physiochemical properties of Cisplatin makes it difficult to produce cisplatin-encapsulated-PLGA controlled release systems in order to maintain sufficient concentration for a longer time[3]. Electrospraying technique is one of the most recent approaches of producing uniform size distribution drug loaded particles with high encapsulation efficiency[4].
Materials and Methods: PLGA solutions (2 and 4wt%) were prepared by dissolving the polymer in DMAc and mechanically stirring for 900 s. Cisplatin at two different concentrations (0.1 and 0.2 wt%) was also added to the solutions. In order to investigate the effect of Cisplatin and PLGA concentration on the size and morphology and eventually the release profile of the particles, three different solutions with various Cisplatin to PLGA ratios (2.5, 5 and 10wt% Cisplatin with respect to PLGA) were electro-sprayed using a single needle EHD setup to produce particles. The solutions were infused via a syringe pump at constant flow rate of 5µl/min, while the voltage was varied from 12 to 15 kV to form a stable cone jet.
Results and Discussion: Particles were electrosprayed whilst PLGA concentration was kept constant at 4 wt%, Cisplatin was increased from 0.1 to 0.2 wt% in the solution. The morphology and size of the particles produced varied at different Cisplatin concentrations (Figure 1). Particles with irregular shapes were produced at higher concentration of Cisplatin (0.2 wt %).
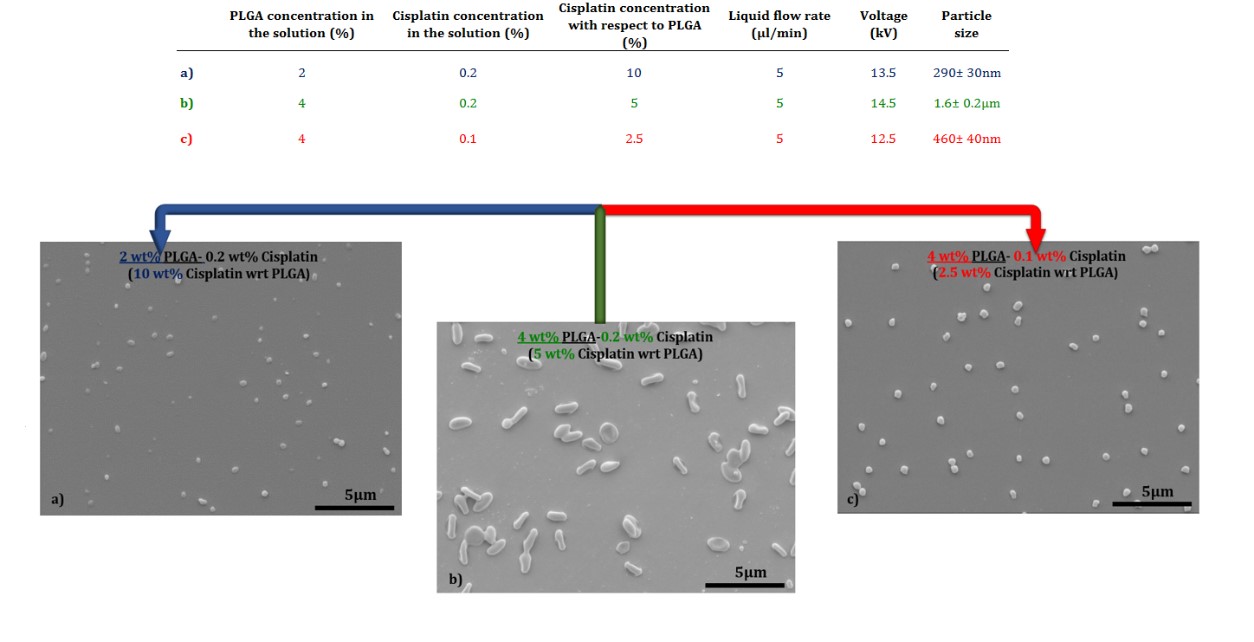
Figure1: SEM images of particles made via EHD technique for different Cisplatin to PLGA ratios of a) 10 wt%, b)5 wt% and c) 2.5 wt%.
Particles were generally larger with the higher Cisplatin concentration (Figure 1b). In order to reduce the size of the particles, the concentration of PLGA was reduced to 2 wt% whilst the higher concentration of Cisplatin (0.2 wt %) was used to increase the ratio of Cisplatin/PLGA. The particles produced were more spherical and became even smaller after the electrospray drying process.
In a separate study, coaxial jets were used to encapsulate Cisplatin in a separate inner layer than PLGA. Cisplatin with 0.1 wt% concentration was encapsulated in the inner Polycaprolacetone (PCL) core and outer PLGA shell. As demonstrated in the TEM images in Figure 2a with encapsulated Cisplatin in the inner layer compared to the same concentration of Cisplatin (0.1 wt%) entrapped in the polymer matrix of the particle produced via the single electrospray jet (Figure 2b).
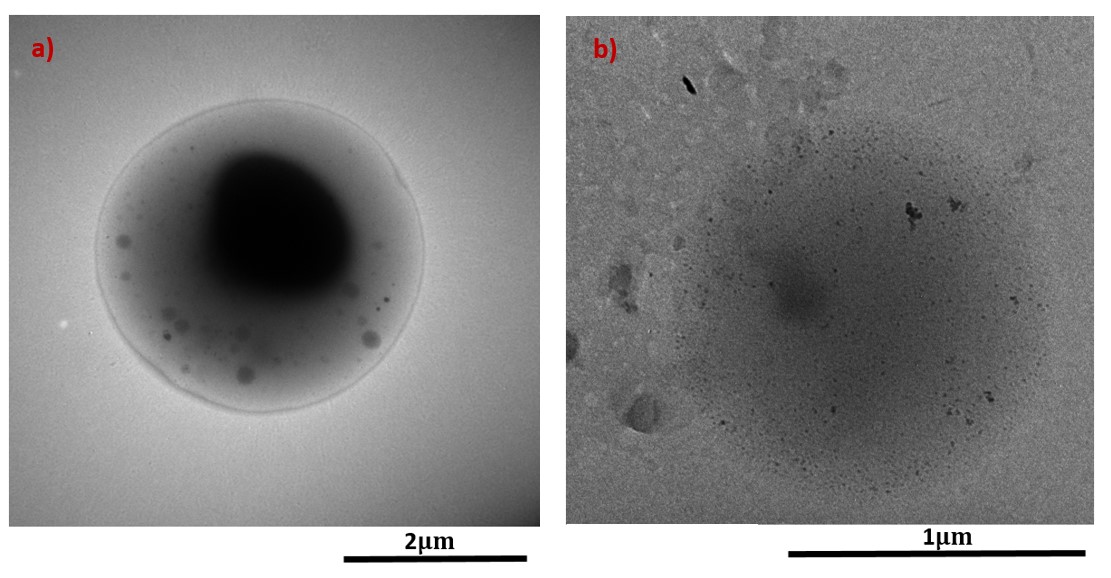
Figure2: TEM images of cisplatin loaded particles obtained with a) coaxial electrospray and b) single needle process for 0.1 wt% Cisplatin concentration.
Conclusion: Cisplatin loaded particles with different morphology and size were produced both with the single and coaxial electrospray jets. The concentration and ratio of Cisplatin to PLGA was varied in order to observe and optimise the particle size and morphology to enhance the drug loading efficiency to achieve a desirable release profile.
The authors would like to thank Engineering and Physical Sciences Research Council (EPSRC) for funding this project under grant number: EP/L025825/1.
References:
[1] Spenlehauer, Gilles, M. Veillard, and J‐P. Benoit. "Formation and characterization of cisplatin loaded poly (d, l‐lactide) microspheres for chemoembolization." Journal of pharmaceutical sciences 75.8 (1986): 750-755.
[2] Dhar, Shanta, et al. "Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles." Proceedings of the National Academy of Sciences 105.45 (2008): 17356-17361.
[3] Moreno, Daniel, et al. "Pharmacodynamics of cisplatin-loaded PLGA nanoparticles administered to tumor-bearing mice." European Journal of Pharmaceutics and Biopharmaceutics 74.2 (2010): 265-274.
[4] Enayati, Marjan, et al. "One-step electrohydrodynamic production of drug-loaded micro-and nanoparticles." Journal of The Royal Society Interface 7.45 (2010): 667-675.