Introduction: Protein adsorption on blood contacting polymers significantly increases the rate of thrombosis and infection on biomedical devices[1]. Here, we have introduced carboxyl groups to polyethylene terephthalate (PET) surfaces using four different functionalization techniques. These carboxylated PET (PET-COOH) surfaces were characterized and their protein antifouling properties were compared.
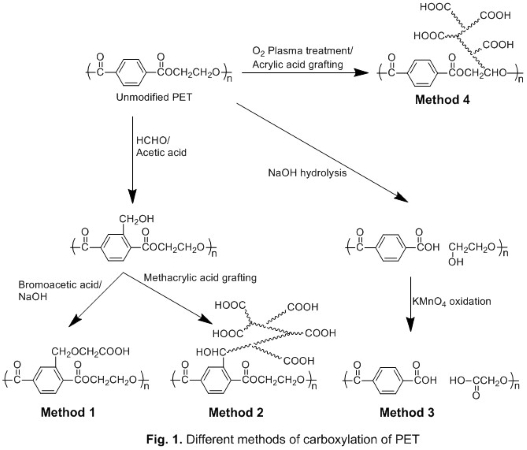
Materials and Methods: Four different methods were used to introduce carboxyl groups on PET surfaces (Fig.1). In method 1 (M1), carboxyl groups were introduced by two step process[2]. In method 2 (M2), initial modifications were same as M1, but methacrylic acid grafting was done on the polymer backbone at the last step[3]. In method 3 (M3), PET was hydrolyzed using NaOH and then functional groups on the surface were oxidized using KMnO4[4]. In method 4 (M4), oxygen plasma treatment was used to introduce reactive peroxide groups on which acrylic acid was grafted[5],[6]. Introduction of carboxyl group was confirmed by fourier transform infrared spectroscopy (FTIR) and estimated using Toluidine blue O (TBO) assay. The surface properties of PET-COOH were characterized by contact angle measurement and scanning electron microscopy (SEM). The bulk property of PET-COOH was characterized using a universal testing machine. Bovine serum albumin (BSA) adsorption on unmodified and modified PET surface was compared using bicinchoninic acid (BCA) assay.
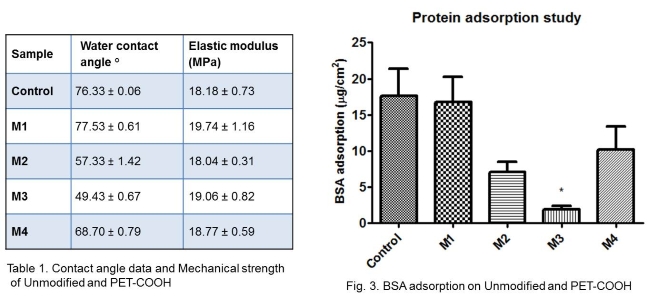
Results and Discussion: Carboxylation was confirmed by the broadening of FTIR peaks in the region 2500-3300 cm-1 on the PET-COOH samples, due to the -OH bond stretching of -COOH group. The amount of carboxyl group estimated by TBO assay for control, M1, M2, M3, M4 was found to be 0.11, 0.31, 12.33, 18.40, 8.74 nmol/cm2, respectively. M3 had maximum carboxylation compared to other methods, which is probably due to the complete oxidation of hydroxyl groups by KMnO4 on the surface. M1 had least carboxylation which is probably due to the less reactivity of aromatic ring for introduction of carboxyl groups. The water contact angles of PET-COOH are lower when compared to unmodified PET (Table 1). These results support the TBO analysis that the hydrophilicity of the modified PET increases with the carboxyl group density on the surface. The SEM images of the modified PET showed that the methods had little or no effect on surface topography, when compared with control (Fig.2).
The elastic modulus of PET-COOH showed no significant changes compared to unmodified PET, suggesting these modifications have no effect on the bulk property (Table 1). The amount of BSA adsorbed on unmodified PET, M1, M2, M3, M4 was found to be 17.56, 16.72, 7.12, 1.87, 10.18 µg/cm2, respectively. M3, having the maximum carboxyl density, shows effective inhibition of protein adsorption (Fig.3). This may be due to the hydration layer formed on the modified surface, which varies depending on the surface carboxyl density[7]. The hydrophilic surfaces forms a very tightly bound water layer creating energy barrier to avoid protein adsorption.
Conclusion: Here, we present a study showing that selection of surface functionalization method is significant for improving the antifouling property of PET even though same functional group are being introduced. The modified PET by M3 was very effective in enhancing antifouling property compared to other methods without altering the bulk property.
We would like to thank A.N.Jeevaraj and Dr. A.Subrahmanyam, Department of Physics, IITM for assisting with the plasma treatment; We also like to thank Department of Biotechnology, Indian Institute of Technology Madras for facilities and fellowship
References:
[1] Banerjee, I.; Pangule, R. C.; Kane, R. S. Advanced Materials. 2011, 690–718.
[2] Yang, Z.; Belu, A. M.; Liebmann-Vinson, A.; Sugg, H.; Chilkoti, A. Langmuir 2000, 16 (19), 7482–7492.
[3] Ma, Z.; Ramakrishna, S. J. Appl. Polym. Sci. 2006, 101 (6), 3835–3841.
[4] Marchand-Brynaert, J.; Deldime, M.; Dupont, I.; Dewez, J.-L.; Schneider, Y.-J. J. Colloid Interface Sci. 1995, 173 (1), 236–244.
[5] Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Vacuum 2009, 84 (1), 83–85.
[6] Gupta, B.; Hilborn, J. G.; Bisson, I.; Frey, P. J. Appl. Polym. Sci. 2001, 81 (12), 2993–3001.
[7] Chen, S.; Li, L.; Zhao, C.; Zheng, J. Polymer. 2010, 51 (23), 5283–5293.