Introduction: Isolated cells or tissues are routinely cryopreserved using slow freezing methods, and stored in banks before therapy. Thawed stem cells should ideally be as effective as fresh ones for patient implantation. However, there is actually no universal and efficient technique to achieve this goal. Cryoprotective agents (CPA) limit ice-crystal damage into cells or tissue[1],[2]. Among CPA, dimethylsulfoxide (DMSO) is the clinical-gold standard, but cytotoxicity has been observed after thawing[3]. Serum is generally used along DMSO, to stabilize cell membranes. Important milestones in cell therapy would be to greatly reduce DMSO concentration, and to discard serum for safety concerns. In this context, polymers represent an alternative to DMSO[4].
However, at high concentrations in solution, osmotic shock could be observed. To overcome this limitation, we have developed CP-gel as CPA, an innovative polysaccharide-based hydrogel technology.
In this study, we evaluated myoblast cryopreservation in presence of CP-gel.
Methods: CP-gel synthesis and characterization. CP-gel was obtained through chemical crosslinking of pharmaceutical grade Dextran as previously described[5], and was characterized (swelling, phosphate content, osmolality) before and after freezing. Temperature of crystallization and quantification of crystallized ice were determined by differential scanning calorimetry.
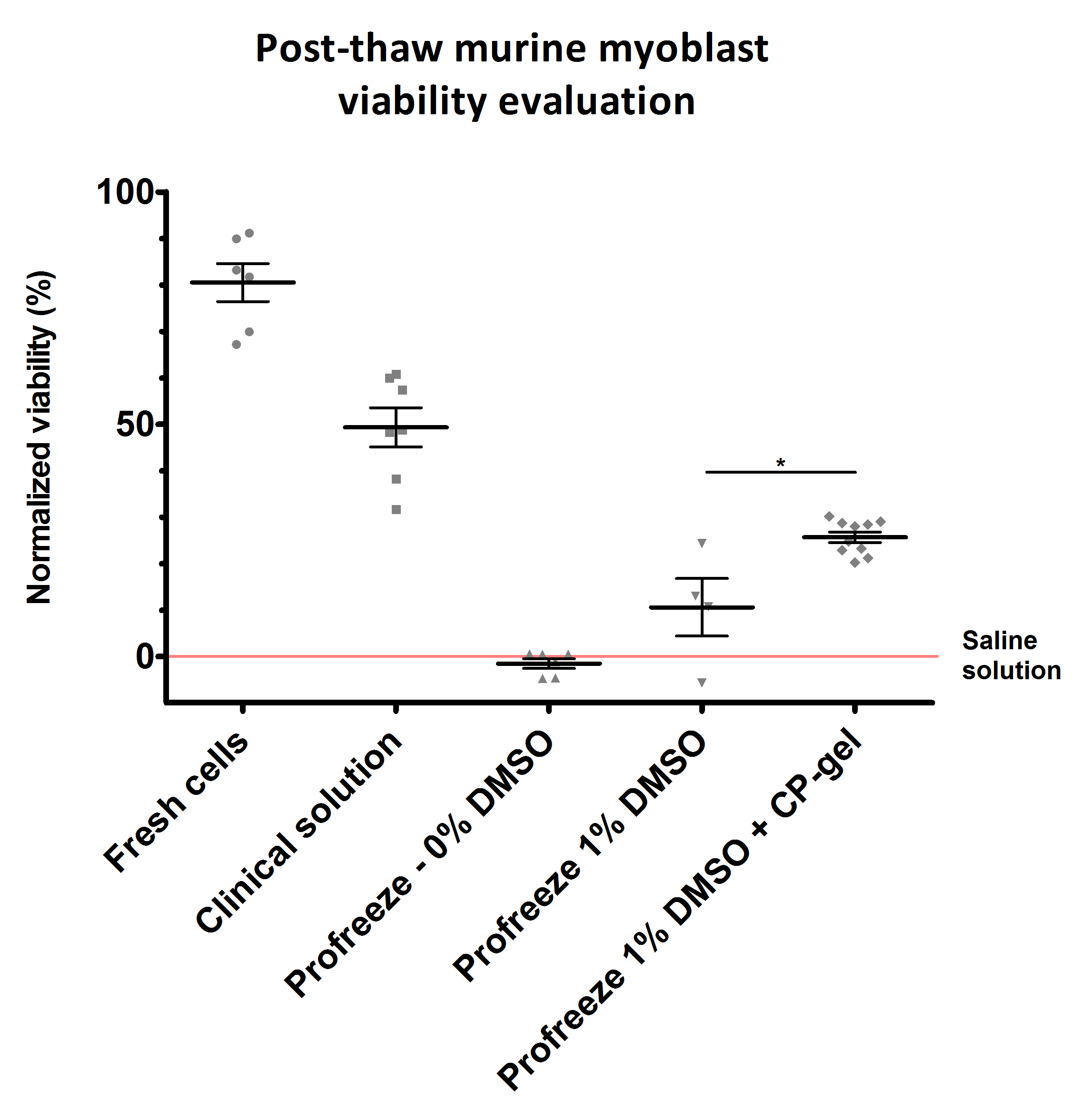
Cell freezing procedure. Primary murine myoblasts were cryopreserved either in HRA-Pharma® clinical solution (Human Serum Albumin 4% + 6% DMSO), in Profreeze™ serum-free freezing medium + 1% DMSO with or without CP-gel, or in Profreeze™ + 0% DMSO and saline solution as negative controls. Slow freezing was performed at -2°C/min with a programmable freezer then samples were stored in liquid nitrogen for 20 days. Cell phenotype (CD56 staining) and viability (AnnexinV & 7-AAD staining) were assessed by flow cytometry, and functionality was evaluated in vitro. Viability% was determined by normalization to saline solution, considered as 0%.
Results and Discussion: Myoblast phenotype was preserved after thawing.
Figure 1 shows that CP-gel addition to Profreeze™ solution significantly improves viability by 15% (p<0.05).
Myoblasts kept their capacity to differentiate when cryopreserved either in clinical solution or Profreeze™ + 1% DMSO + CP-Gel (Figure 2, red arrows).
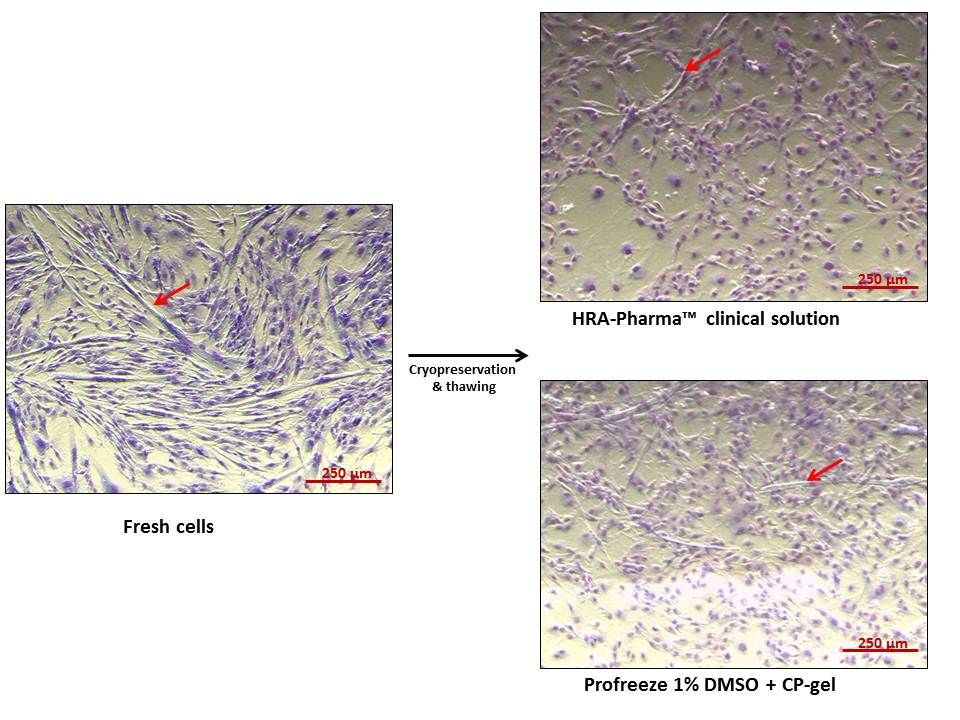
Figure 2: Differentiation of myoblasts before and after cryopreservation. Red arrows point myotube formation. Giemsa staining.
These results suggest that CP-gel could improve cryopreservation in serum-free solution, and can lower DMSO concentrations, therefore reducing cytotoxicity or clinical side-effects[6] and help go through cell therapy regulation. Furthermore, CP-gel allowed an easy cell recovery without enzymatic digestion[7].
Conclusion: By its properties, CP-gel could be used as a new toolkit for improving cells or tissues biobanking.
Murine myoblasts studies gave us clues to tackle human adult myoblast cryopreservation with CP-gel, wich will is currently investigated in the laboratory.
References:
[1] Kuleshova, L. and D. Hutmacher, Chapter 13 - Cryobiology, in Tissue Engineering, C.v. Blitterswijk, et al., Editors. 2008, Academic Press: Burlington. p. 363-401.
[2] Meryman, H.T., Cryopreservation of living cells: principles and practice. Transfusion, 2007. 47(5): p. 935-45.
[3] Fahy, G.M., Cryoprotectant toxicity neutralization. Cryobiology, 2010. 60(3, Supplement): p. S45-S53.
[4] Deller, R.C., et al., Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. 2014(2041-1723 (Electronic)).
[5] Abed, A., et al., Influence of polysaccharide composition on the biocompatibility of pullulan/dextran-based hydrogels. 2011(1552-4965 (Electronic)).
[6] Morris, C., et al., Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. 2014(1537-2995 (Electronic)).
[7] Camboni, A., et al., Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. 2013(1090-2392 (Electronic)).