Introduction: The culture of various cell types including induced pluripotent stem cells (iPSC), requires the use of growth factors such as bFGF (basic Fibroblast Growth Factor)[1],[2]. These factors are generally added to the medium in their diffusive form and have a very short half-life[3]: they disappear quickly from the culture medium and must be replaced at high cost. We here explore the stable and oriented tethering of bFGF on a cell culture substrate, namely gelatin (denatured collagen). Our strategy relies on the expression of two fusion proteins being labelled with two distinct peptides (the E and Kcoils) that bind to each other with great affinity and specificity[4]. That is, a chimera corresponding to bFGF fused to the Ecoil peptide on the one hand, and the collagen-binding domain (CBD) of fibronectin fused to the Kcoil peptide on the other hand (Fig. 1).
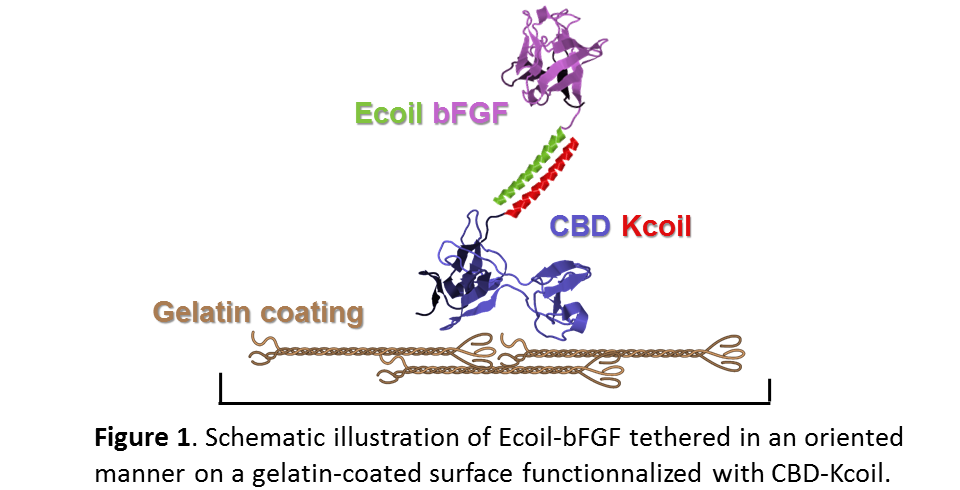
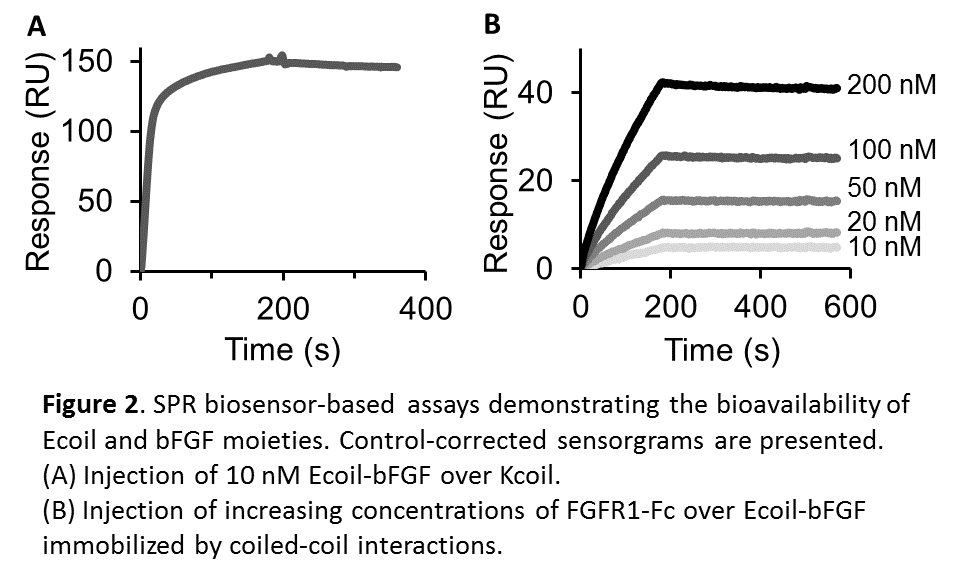
Materials and Methods: The Ecoil-bFGF fusion protein was produced in E. coli and purified by Ni-NTA affinity chromatography followed by TEV protease cleavage and ion exchange chromatography. The CBD-Kcoil fusion protein was produced in E. coli and purified by solubilizing inclusions bodies in an 8 M urea buffer, dialyzing against PBS, and then followed by a Ni-NTA affinity chromatography. The ability of Ecoil-bFGF protein to bind to Kcoil was assessed by surface plasmon resonance (SPR), using a Kcoil-coated chip while its ability to bind to its cognate receptor was tested by subsequent injections of a recombinant chimera of the FGF Receptor (FGFR1-Fc). Finally, the suitability of the proposed approach for cell culture will be soon assessed by cultivating iPSCs on a gelatin-coated substrate to be functionalized with CBD-Kcoil/Ecoil-bFGF.
Results and Discussion: Both fusion proteins were produced and purified with high purity. The yield of the purification of Ecoil-bFGF was assessed by ELISA and reached 1.1 mg per liter of bacteria. On the one hand, SPR analyses (Fig. 2) demonstrated that Ecoil-bFGF specifically bound to a Kcoil-coated chip, and subsequently interacted with FGFR1-Fc with high affinity (KD = 1.8 nM), in good agreement with the literature[5]. On the other hand, the bioactivity of CBD-Kcoil was assessed via an Enzyme-Linked Immunosorbent Assay (ELISA) against bFGF: gelatin-coated substrates were incubated with CBD-Kcoil then Ecoil-bFGF, and the formation of the gelatin/CBD-Kcoil/Ecoil-bFGF complex was demonstrated by the specific recruitment of anti-bFGF antibodies. Altogether, the data indicated that our novel strategy enabled the specific capture of bioactive bFGF via collagen/CBD and coiled-coil interactions.
Conclusion: Providing iPSCs with bioactive bFGF in a non-diffusible form may significantly decrease expenses related to cell culture. Furthermore, the modular approach we undertook may make this strategy amenable to the development of more complex scaffolds combining several growth factors (bFGF, EGF, VEGF ...), and thus pave the way to the development of novel approaches in the field of tissue engineering.
This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), CREATE program - MEDITIS scholarship.
References:
[1] M. Amit, M. K. Carpenter, M. S. Inokuma, C.-P. Chiu, C. P. Harris, M. A. Waknitz, J. Itskovitz-Eldor, and J. A. Thomson, “Clonally Derived Human Embryonic Stem Cell Lines Maintain Pluripotency and Proliferative Potential for Prolonged Periods of Culture,” Dev. Biol., vol. 227, no. 2, pp. 271–278, Nov. 2000.
[2] K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, and S. Yamanaka, “Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors,” Cell, vol. 131, no. 5, pp. 861–872, Nov. 2007.
[3] G. Chen, D. R. Gulbranson, P. Yu, Z. Hou, and J. A. Thomson, “Thermal Stability of Fibroblast Growth Factor Protein Is a Determinant Factor in Regulating Self-Renewal, Differentiation, and Reprogramming in Human Pluripotent Stem Cells,” Stem Cells Dayt. Ohio, vol. 30, no. 4, pp. 623–630, Apr. 2012.
[4] F. Murschel, B. Liberelle, G. St-Laurent, M. Jolicoeur, Y. Durocher, and G. De Crescenzo, “Coiled-coil-mediated grafting of bioactive vascular endothelial growth factor,” Acta Biomater., vol. 9, no. 6, pp. 6806–6813, Jun. 2013.
[5] X. Lin, K. Takahashi, S. L. Campion, Y. Liu, G. G. Gustavsen, L. A. Peña, and P. O. Zamora, “Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo,” Int. J. Mol. Med., vol. 17, no. 5, pp. 833–839, May 2006.