Introduction: Hydrogels constructed from zwitterionic poly(carboxybetaine) (pCB)-based polymers and crosslinkers offer unique advantages for tissue engineering applications. Because of their strong hydration and resistance to nonspecific interactions, they can act as growth scaffolds to maintain stem cell pluripotency or precisely direct their fate[1], mitigate the foreign body reaction to implanted materials[2] and demonstrate self-healing abilities[3]. In this work we have developed a click-chemistry-based platform for the in situ formation of pCB hydrogels under physiological and cytocompatible conditions.
Materials and Methods: A copolymer of carboxybetaine (CB) monomer and a modified CB monomer containing a protected thiol moiety is synthesized using RAFT polymerization. After thiol deprotection, this copolymer is mixed with a disulfide-containing carboxybetaine crosslinker to initiate gelation through thiol-Michael addition in physiological conditions. This process is illustrated in the figure. Cells, biologics or drug carriers can be entrapped in the matrix during gelation by seeding them in the polymer solution immediately before crosslinker addition.
Preliminary Results: Thiol-modified CB monomer and degradable disulfide CB crosslinker were synthesized. Gel formation in situ was observed upon mixing copolymer and crosslinker solutions, and this formed hydrogel was confirmed to be non-toxic to several cell lines. Drug-loaded poly(lactic-co-glycolic acid) (PLGA) microparticles could be entrapped in the gel during formation (as shown in the figure) and recovered when the gel was deliberately degraded with DTT. Cell encapsulation and growth studies are under way.
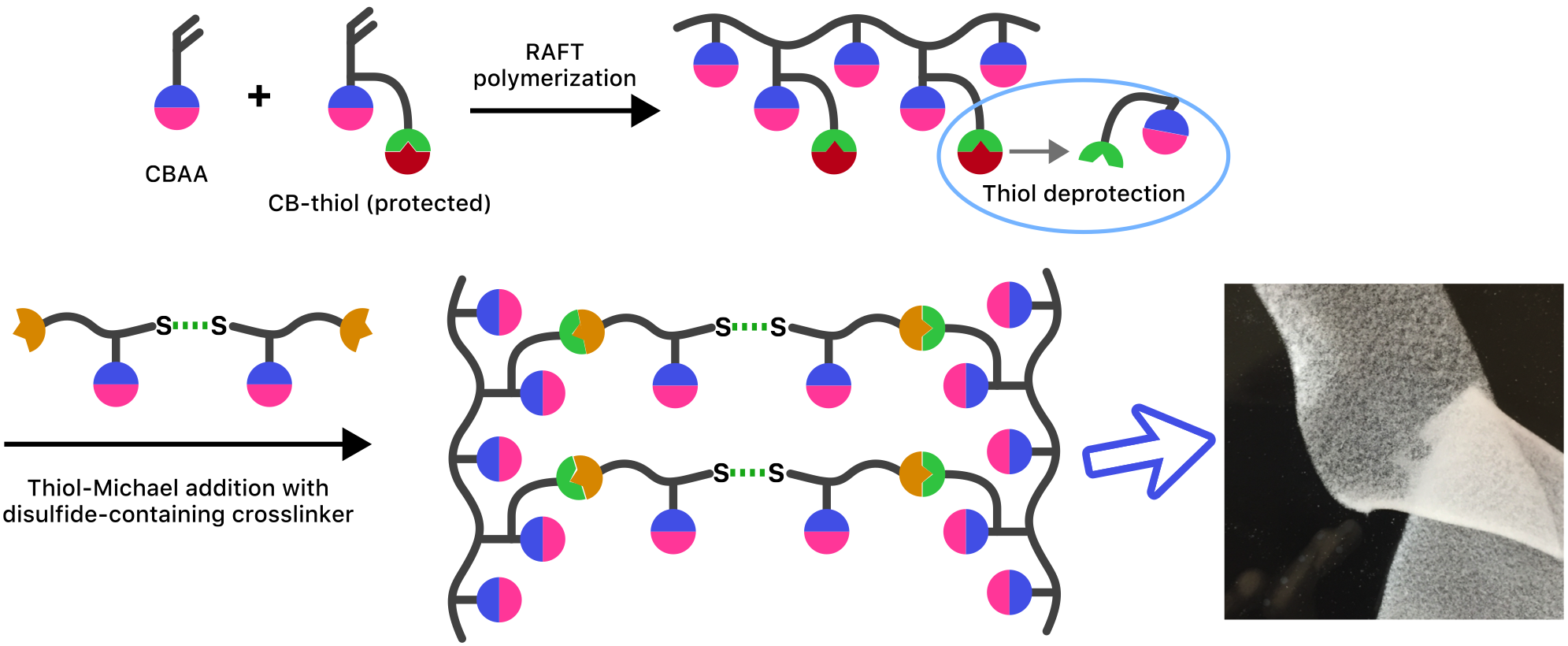
Discussion: Of all available hydrogel-based biomaterials, the unique ability of pCB hydrogels to strongly resist nonspecific interactions makes them especially suited for tissue scaffolds and implanted materials for cell therapy or drug delivery. Thiol-click chemistry enables these hydrogels to be formed under physiological conditions without adding initiator or inducing radical polymerization, which expands their applications to sensitive cell lines and injectable scaffolds.
This research was funded by the National Science Foundation (DMR 1307375 and CBET-1264477)
References:
[1] T. Bai, F. Sun, L. Zhang, A. Sinclair, S. Liu, J.R. Ella-Menye, Y. Zheng and S. Jiang, "Restraint of the Differentiation of Mesenchymal Stem Cells by a Nonfouling Zwitterionic Hydrogel", Angewandte Chemie. Vol. 53, pp 12729-12734 (2014)
[2] L. Zhang, Z. Cao, T. Bai, L. Carr, J.R Ella-Menye, C. Irvin, B. Ratner, S. Jiang, "Zwitterionic hydrogels implanted in mice resist the foreign-body reaction", Nature Biotechnology. Vol. 31, pp 553-556 (2013)
[3] T. Bai, S. Liu, F. Sun, A. Sinclair, L. Zhang, Q. Shao and S. Jiang, "Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions", Biomaterials. Vol. 35, pp 3926-3933 (2014)