Introduction: Magnetic nanoparticles (MNPs) and calcium phosphates, are versatile nanomaterials increasingly adopted in the biomedical research. MNPs are being currently investigated for their therapeutic potentialities in anticancer hyperthermic treatments. Particularly, in bone cancer, the necessity of a bone replacement after osseous excision, and contemporary of a local anti-tumor treatment would require materials which could perform both these functions. Bearing this in mind, we intended to investigate MNPs-decorated nanohydroxyapatite (n-HA) as putative agents against bone cancer. The goal of this study is in fact to synthesize bone–mimicking HA nanocrystals able to show magnetic responsiveness and which can act as both bone fillers and anticancer hyperthermic agents.
Materials and Methods: Dextran-grafted Fe3O4 nanoparticles (DM) have been prepared by an easy and scalable synthesis based on a previously reported method by Walsh et al., 2007. Then, magnetic nano-architectures based on n-HA decorated by DM nanoparticles (DM-HA) have been synthesized by an aqueous precipitation technique in the presence of different amounts of DM (i.e. DM-HA weight ratio: 1/1, 2/1, 3/1). Morphological, compositional and magnetic properties of nanocrystals were investigated by XRD, TEM, ICP-AES, FTIR techniques and a Susceptometer. Finally, effects of nanoparticles on human bone-derived cell lines (MG63) were evaluated by MTT proliferation assay and by differential staining of cell compartments.
Results and Discussion:
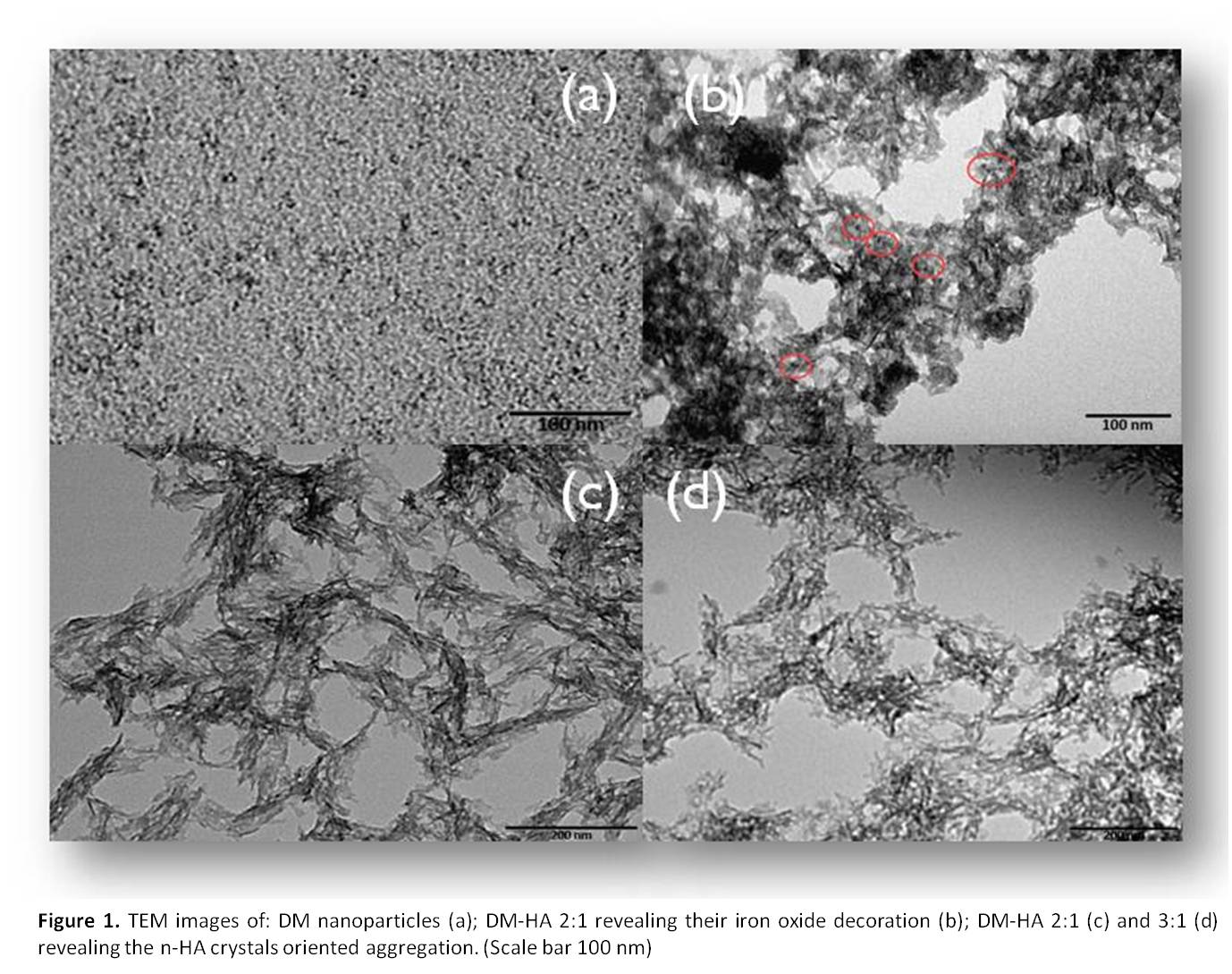
TEM images of DM nanoparticles reveal size mono-dispersion, with particle diameters ranging between 2 and 5 nm (Fig. 1(a)), whereas TEM analysis of DM-HA nano-architectures shows plate-like n-HA crystals surface-decorated by DM nanoparticles (Fig. 1(b)). Moreover plate-like n-HA crystals seem to aggregate along one direction in the presence of DM nanoparticles (Fig. 1(c,d)). For each DM-HA ratio X–ray diffraction patterns are consistent with single-phase low crystalline HA similar to deproteinized bone apatite. Magnetic susceptibility of DM-HA shows modulation of the magnetization as a function of the DM content.
Cytocompatibility parameters of DM-HA were assessed by MG63 and confirm that, the bioactivity of this material is not affected by the amount of DM used. Morphological and physical interactions between DM-HA and cells were evaluated and compared to DM and n-HA alone: no significant changes in cell morphology were observed in the absence of a magnetic field.
Conclusions: The magnetic behavior of these nano-architectures, allowed to establish that every properties connected to their magnetization, can be switched by external stimuli. Furthermore, their cytocompatibility towards bone-derived human cell line in the absence of a magnetic field, renders them able to be used as bone substitutes, and as a potential “on demand” local hyperthermic agents.
MIUR (PON RINOVATIS No.02_00563_3448479)
References:
[1] Walsh D et al., Biomacromolecules 8:3800-3805, 2007