- 1Department of Chevidence Lab Child & Adolescent Health, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2Ministry of Education Key Laboratory of Child Development and Disorders, National Clinical Research Center for Child Health and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3Chongqing Key Laboratory of Pediatrics, Chongqing, China
- 4Department of Pharmacy, Gansu Provincial Hospital, Lanzhou, China
- 5Institute of Health Data Science, Lanzhou University, Lanzhou, China
- 6Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 7School of Public Health, Lanzhou University, Lanzhou, China
- 8Research Unit of Evidence-Based Evaluation and Guidelines, Chinese Academy of Medical Sciences (2021RU017), School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 9WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
- 10GRADE Center, Lanzhou University, Lanzhou, China
- 11Department of Nephrology, Children's Hospital of Chongqing Medical University, Chongqing, China
Objective: To systematically analyze the supporting evidence, drug information, and the type of off-label drug use in recommendations on off-label drug use in pediatric guidelines.
Methods: A cross-sectional study was performed by systematic search through MEDLINE (via PubMed) and Embase databases to identify literature published from 1 January 2018, to 31 December 2020. Only pediatric clinical practice guidelines that included recommendations on off-label use of drugs were included. We present descriptive information on the sources of the included guidelines, country, publication year, evidence grading system used, details on the types of off-label drug use, and the types of studies used as references to support the recommendations.
Results: A total of 66 pediatric guidelines with 605 recommendations were included. Eighty-seven (14.4%) recommendations did not cite any references; and the remaining 518 recommendations were supported by 2,240 references (mean 4.3 references/recommendation). The most common types of studies cited were pediatric RCTs (n = 314, 14.0%), pediatric case series studies (n = 260, 11.6%), and reviews (n = 255, 11.4%). Twenty-one percent (n = 470) of the references were studies on adults. One hundred and forty (23.1%) recommendations were graded using the Grading of Recommendations, Assessments, Development, and Evaluations (GRADE) system, of which 37 (26.4%) were graded as strong but supported with only C or D level of evidence. The most commonly reported type of information in the recommendations was indication (n = 499, 82.5%). The most commonly addressed type of off-label drug use in the 523 positive recommendations was unapproved population (n = 255, 48.8%). Sixty-nine (11.4%) recommendations explicitly reported the drug use as off-label.
Conclusion: Children may be exposed to medical risks due to gaps in reporting and evidence of off-label drug use recommendations in pediatric guidelines.
Introduction
Off-label use of drugs is highly common in pediatrics (Schrier et al., 2020; Sweileh, 2021) due to delays in updating drug instructions and difficulties in conducting clinical trials (Hudgins et al., 2018; Hwang et al., 2018; Wu et al., 2019; Carmack et al., 2020). The prevalence of pediatric off-label drug prescriptions has been estimated to range from 3.2% to 95% overall, 26%–95% in neonates (Allen et al., 2018; Balan et al., 2018), 2.7%–51.2% in outpatients, and 9.0%–79.0% in inpatients (Li et al., 2016; Zhou Y. et al., 2021). However, sometimes off-label use of drugs could be inappropriate or without proven therapeutic benefit. Medical decisions involving off-label drug use should thus be based on the existing evidence (Frattarelli et al., 2014; Ito, 2017) to avoid irrational medication use. Clinical Practice Guidelines (CPGs) that aim to collect, grade and summarize the latest available evidence are the best option for mitigating the risk of irrational pharmaceutical use and the liability associated with the off-label use of drugs (guidelines., 2011).
At present, there are gaps in the reporting and evidence of off-label drug recommendations in pediatric guidelines. The lack of evidence for pediatric off-label use of drugs raises concerns about efficacy and safety. Some guidelines did not mention that the recommended treatment was off-label, potentially complicating clinical practice (Kochanek et al., 2019). In addition, there is often substantial variation in the information reported between different guidelines that recommend the same drug for off-label use (Zhou P. et al., 2021). Indications, dosage, cautions, medication regimen, side effects, pharmacological mechanisms, and drug interactions are all included in the guidelines of specific drugs. However, guidelines not focusing on any specific drug are usually less comprehensive in reporting drug related information. Moreover, there are more adverse drug reactions associated with off-label prescribing that are not supported by good evidence (Eguale et al., 2016). As a result, there is a need to better clarify the current evidence for pediatric off-label drug use in pediatric guidelines to assist in the judicious use of drugs.
This study focuses on recommendations on off-label drug use in pediatric guidelines published in the last 3 years, with the aim to demonstrate the variability in reporting recommendations, present information on drugs recommended for off-label use for different disease categories, and analyze the evidence on off-label drug use in pediatrics. Our study will provide a reference for guideline developers to present recommendations on off-label use of drugs and for pediatricians to guide the off-label administration of drugs.
Methods
Study Design
A cross-sectional study was conducted.
Search Strategy
We performed a systematic search through MEDLINE (via PubMed) and Embase databases to identify literature published from 1 January 2018, to 31 December 2020, with the terms “infant,” “newborn*,” “adolescent,” “child*,” “pediatric*,” “guideline*,” and “recommendation*” (Supplementary Table S1).
Inclusion and Exclusion Criteria
We included articles that met the following criteria: 1) the article was a guideline published in an academic journal between 2018 and 2020; 2) the guideline addressed exclusively pediatric clinical practice; and 3) the guideline contained recommendations on off-label use of drugs. Duplicates, guidelines that included adults, translated guidelines, executive summaries, or articles published in languages other than English were excluded.
Study Selection
Retrieved records were exported into Endnote X9 (version 9.3.1). Six investigators were divided into three groups (Group 1: Min Meng & Ping Wang, Group 2: Yunlan Liu & Wenjuan Lei, and Group 3: Min Tian & Yajia Sun). The identified records were divided between the three groups. In all groups both investigators independently screened first the titles and abstracts of the identified records, and then the full texts of the potentially eligible articles. Disagreements were solved by consensus or consultation with the senior investigators.
Data Extraction
Two pharmacists with experience in clinical pharmacy independently extracted the information in the guidelines containing recommendations related to the off-label use of drugs. For pediatric guidelines with clear recommendations, the information on the off-label drug was further analyzed. The extracted information included: 1) basic information: publisher (organization), publication time, target population, disease category, disease (Pocai, 2019) (ICD-11 classification), number and content of recommendations, number of positive and negative recommendations (see definition below), used evidence grading systems, and conflict of interests (Supplementary Table S2); 2) Information on off-label drug use: content of the recommendations, name of the drug (or drug class, depending on whether a specific drug or a class of drugs is mentioned in the recommendation), details of the drug administration (indication, route of administration, dose, precise dosage, course of treatment, precautions, adverse reactions, contraindications, others), types of off-label drug use (see categorization in the next paragraph), types of cited literature, content of the evidence, and the level of recommendation according to the GRADE grading system (Supplementary Table S3) (Atkins et al., 2004a).
A negative recommendation was defined as a recommendation using unambiguously negative terms, such as “no,” “against,” “avoid,” or “contraindication.” A suggestion was considered positive if it contains both positive and negative descriptions. The types of off-label use were categorized into the following groups (Aronson and Ferner, 2017): 1) unapproved indication: the drug was recommended for a condition not mentioned in the drug instructions; 2) unapproved age: the drug was recommended for children outside the age range specified in the instructions; 3) unapproved population group: the drug was approved for the indication in adults but not in children; 4) unapproved route of administration; 5) unapproved dosage or frequency: dose and frequency inconsistent with the description in the instructions; or 6): unlicensed: the drug or hospital preparation was not approved or was withdrawn, by the US Food and Drug Administration (FDA). The FDA instructions and the Micromedex pharmaceutical information system were used to determine the drug class and type of off-label use (https://www.micromedexsolutions.com/). If a pharmaceutical could be classified into multiple drug classes, we determined the most appropriate drug class based on the ailment addressed in the guidelines (Supplementary Table S3).
Two investigators independently extracted the data and conducted two pilot rounds to improve consistency. Discrepancies were resolved through consultation with the senior investigators.
Data Analysis
A descriptive statistical analysis of the included studies was performed, which presents the characteristics of the guidelines and information on off-label drugs. The distribution of reference types for off-label drug recommendations, drug ranking (highlighted drugs are on the WHO Model List of Essential Medicines for Children - 8th list, 2021 (WHO, 2021b)), and drug classes were also studied descriptively. Excel 2021 was used to collect and analyze the data (version 16.54).
Results
Characteristics of Included Guidelines
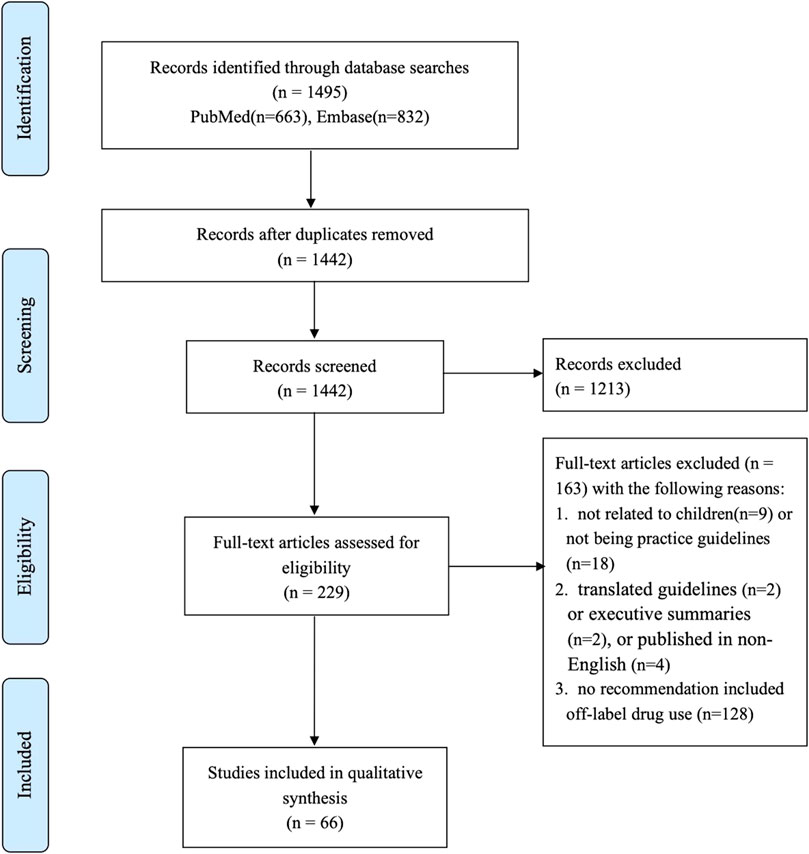
We identified 1,465 articles through the electronic database searches, and 66 guidelines (containing 605 recommendations) were finally included after the title/abstract and full-text screening. The full process of the literature search is shown in Figure 1.
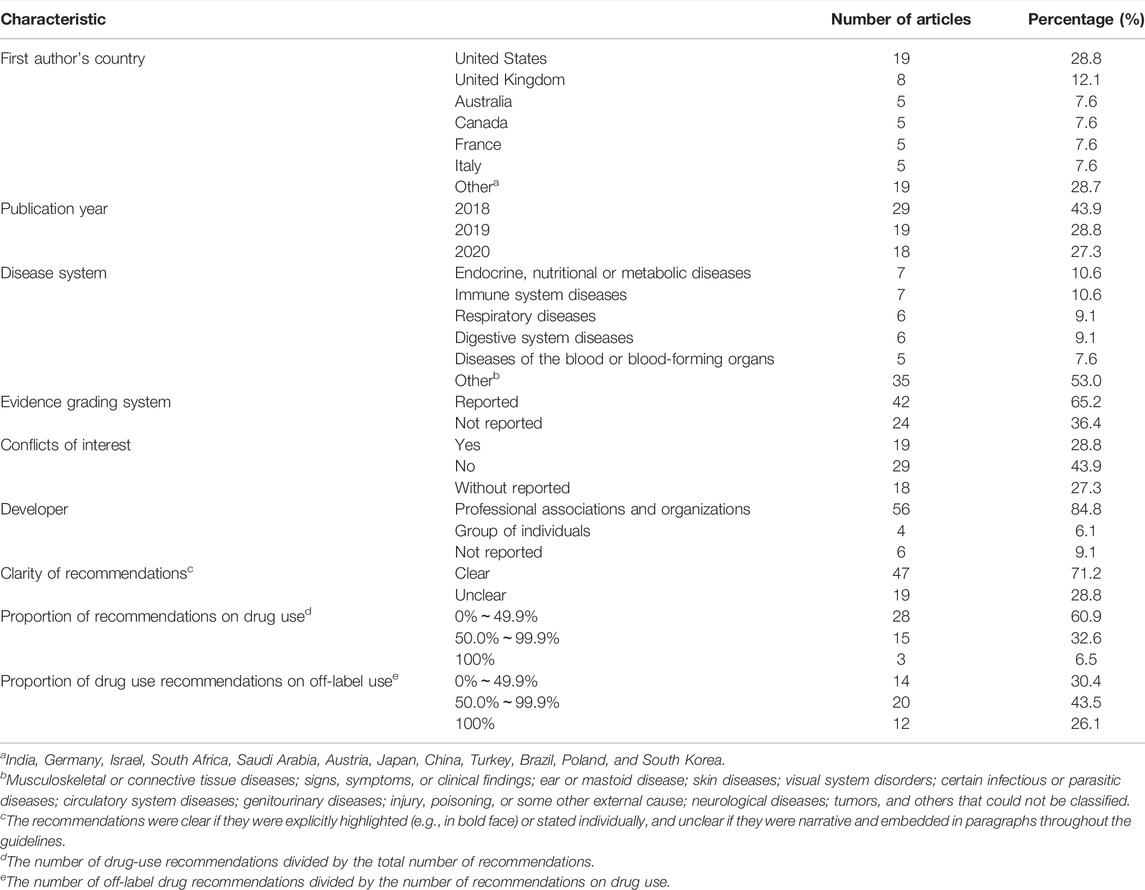
The first authors of the 66 guidelines were from 18 countries, with nearly one-third from the United States (n = 19, 28.8%), and more than 80% (n = 58, 87.9%) from high-income countries. A grading system for evidence was adopted in 42 (63.6%) guidelines, conflicts of interest was declared in 19 (28.8%) guidelines, with 9 (13.6%) guidelines may had commercial involvement in the recommendation of off-label use, and 56 (84.8%) guidelines were produced by professional societies or organizations.
Endocrine, nutritional, or metabolic disorders (n = 7, 10.6%), and immune system diseases (n = 7, 10.6%) were the most frequently addressed disease systems. The number of recommendations could not be counted in 20 (30.3%) guidelines because of the ambiguousness of the recommendations. Forty-six (69.7%) guidelines had clear recommendations, of which twelve only discussed drugs. Table 1 lists the characteristics of the included guidelines.
Types of References for Recommendations on Off-Label Drug Use
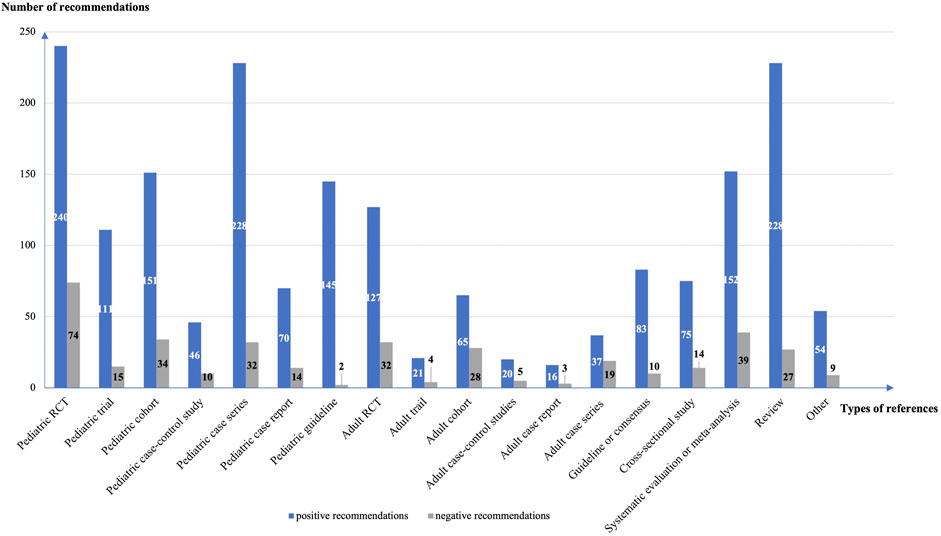
The guidelines contained a total of 605 recommendations on off-label drug use, including 523 positive (86.4%) and 82 negative (13.6%) recommendations. Eighty-seven (14.4%) recommendations had no references. We analyzed 1869 references for the 441 positive recommendations with references, and 371 references for 77 negative recommendations with references. The mean number of references per recommendation was 4.3 (4.2 for positive recommendations and 4.8 for negative recommendations). Pediatric randomized controlled trials (n = 314, 14.0%), pediatric case series (n = 260, 11.6%), and reviews (n = 255, 11.4%) were the most common types of reference (Figure 2). A total of 470 (21.0%) references were studies from adults.
Grading of Evidence Supporting the Recommendations on Off-Label Drug Use
The GRADE evidence grading system was used in 140 (23.1%) of the 605 recommendations for off-label use of drugs. The quality of evidence was GRADE level D in 70 (53.4%), GRADE level C in 47 (35.9%), GRADE level B in 13, 9.9%), and GRADE level A in 1 (0.8%) study. There were more weak (n = 86, 65.6%) than strong (n = 45, 34.4%) recommendations, including 37 (26.4%) strong recommendations supported with GRADE C or D level evidence (Figure 3).
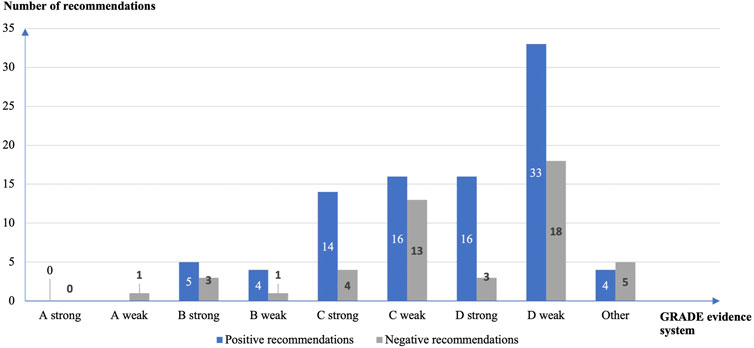
FIGURE 3. Strength of the off-label drug use recommendations and quality of the supporting evidence according to the GRADE grading system.
Information on Off-Label Drug Use
The content and extent of the information reported varied considerably between the recommendations on off-label drug use. The indication was reported in 499 (82.5%), route of administration in 253 (41.8%), dosage in 183 (30.2%), precise dosage in 128 (21.2%), precautions in 175 (28.9%), adverse reactions in 88 (14.5%), course of treatment in 78 (12.9%), contraindications in 30 (5.0%) and other information in 60 (9.9%) recommendations.
Types of Off-Label Drug Use
Sixty-nine (11.4%) of the 605 assessed recommendations clearly and explicitly stated the use of the drug as off-label. Among the 523 positive recommendations, 255 (48.8%) addressed use for unapproved population, 201 (38.4%) for unapproved indication and 185 (35.4%) for unapproved age, 20 (3.8%) the use of unlicensed drugs, and 15 (2.9%) drug use with unapproved dosage or frequency and 7 (1.3%) with unapproved route of administration.
Ranking of Specific Off-Label Drugs and Drug Types in the Recommendations
We obtained a total of 941 drug names (652 specific medicines and 289 drug classes) from the 605 off-label drug recommendations. The three most commonly mentioned individual drugs were acetaminophen (n = 31, 3.3%), fentanyl (n = 28, 3.0%) and ropivacaine (n = 24, 2.6%). The three most commonly mentioned drug classes were nonsteroidal anti-inflammatory drugs (NSAIDs; n = 46, 4.9%), low molecular weight heparins (LMWHs; n = 28, 3.0%), and anticoagulants (n = 24, 2.6%) (Supplementary Table S4).
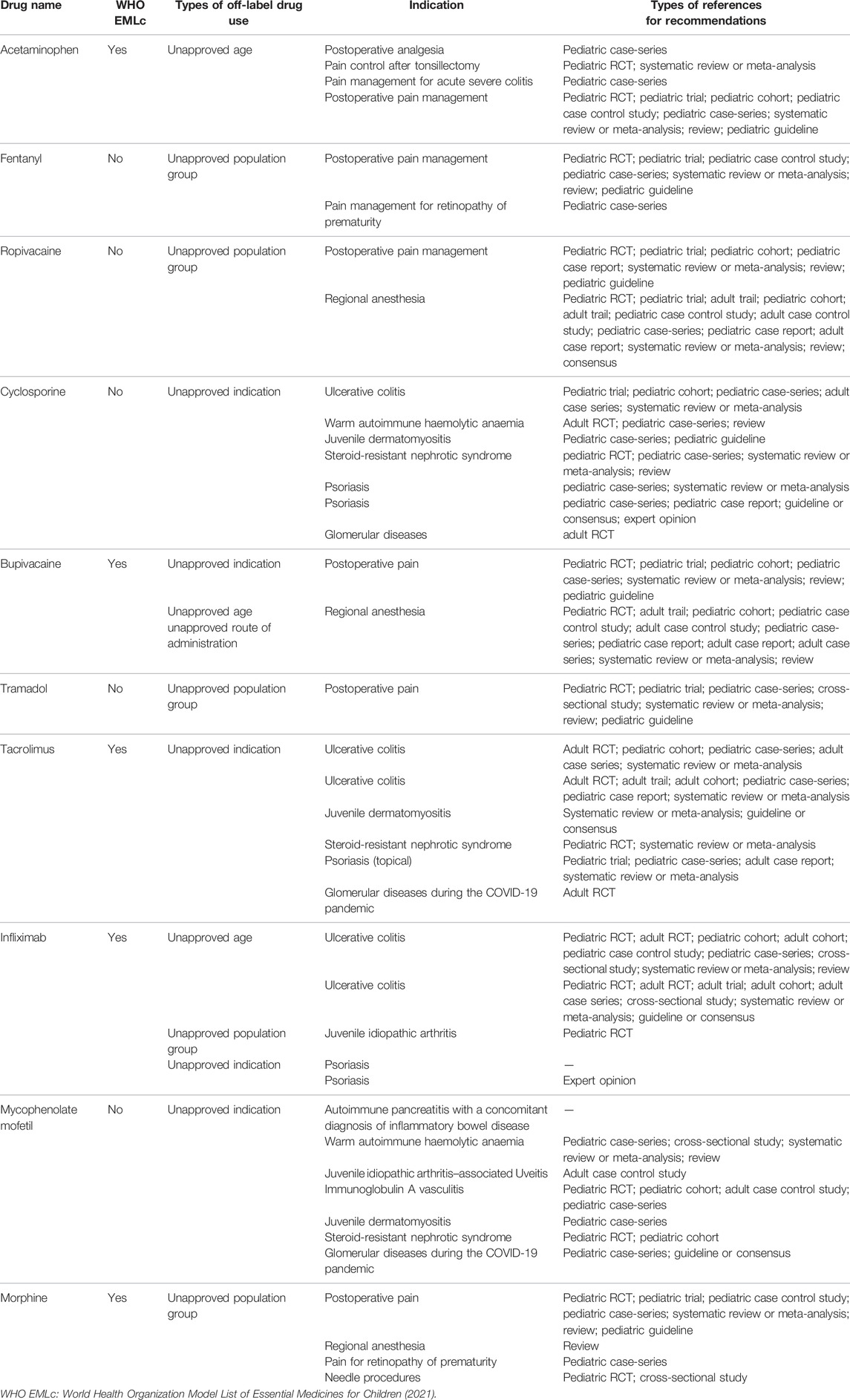
We analyzed detailed recommendations of the top ten off-label drugs (Table 2), of which five drugs were not on the World Health Organization Model List of Essential Medicines for Children. There was fentanyl, ropivacaine, cyclosporine, tramadol, and mycophenolate mofetil. Moreover, there were no references to support the use of infliximab for psoriasis and mycophenolate mofetil for pancreatitis with autoimmune pancreatitis in children, respectively.
Recommendations for Acetaminophen or Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Acetaminophen and NSAIDs were the most commonly mentioned drug and drug classes, respectively. Positive recommendations on these drugs provided detailed information on the use of the medication, whereas negative recommendations only addressed the indications that are not suggested (Table 3). The contents of drug information descriptions were diverse among the different diseases, and we could not identify any pattern. The recommendation on NSAIDs for post-tonsillectomy pain control reported the most detailed drug information, including indications, route of administration, dose, precise dosage, adverse effects, and contraindications.
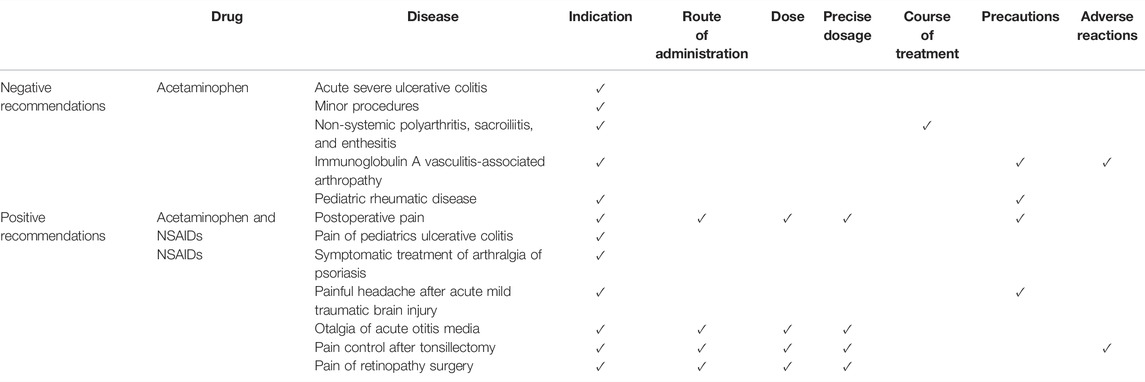
TABLE 3. Diseases and information covered by the recommendations on off-label use of nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen.
Discussion
Main Findings
This study identified 605 recommendations on off-label drug use from 66 pediatric guidelines. About 90% of the recommendations did not explicitly designate their recommended drugs as off-label use. Various types of references were cited to support the recommendations, with more than one-fifth being adult studies and 15% of the recommendations lacking references. More than a quarter of the recommendations that were graded using the GRADE evidence system were classified as strong but based on low or very low quality evidence.
Evidence Supporting the Recommendations on Off-Label Drug Use in Pediatric Guidelines
The majority of clinical decisions are based on the principles of evidence-based medicine (Djulbegovic and Guyatt, 2017). However, in many cases, only low-quality evidence or evidence from adult populations is available to inform the treatment of children. For example, to support off-label pharmacological use in neonates, case reports (Tobin, 2010), expert opinions, and evidence extrapolated from data on other populations are frequently used (Slater et al., 2020). When a new drug is launched, evidence is first usually only available on adults. In the early phases of a public health emergency, only low-level evidence, such as case series, is available (Hudgins et al., 2018; Lagler et al., 2021). Treatment of psychiatric disorders in children is often based on evidence from adults (Sharma et al., 2016), and only case reports are often available for rare diseases (Whitley and Chepke, 2020). As a result, strong recommendations are sometimes generated based on expert consensus (Rosen et al., 2018) and indirect evidence of similar diseases (Liu et al., 2020), as well as based on conclusions from disease features and clinical practice experience. However, the inconsistency between the quality of the evidence and the strength of the recommendation may contradict the critical principles of evidence-based medicine, creating a danger to clinical practice (Yao et al., 2021). As a result, it is critical that recommendations on the off-label use of drugs in pediatric guidelines are based on a reliable methodological pathway or framework (Cheng et al., 2007). However, there is currently no standardized framework for creating recommendations for the use of off-label drugs in children (Casali et al., 2015; Agrawal et al., 2019; Jiang et al., 2020; Stolbach et al., 2020; Remi et al., 2021). In general, the development of clinical guidelines should follow the GRADE Working Group’s “Five Paradigmatic Situations Warranting Strong Recommendation Despite Low or Very Low-Quality Evidence in Effect Estimates,” which has proven to offer a reliable framework (Andrews et al., 2013). This framework, unfortunately, does not resolve the clinical aspects regarding off-label use of drugs in children, and the interpretation and judgments of the GRADE framework may vary between individuals. Furthermore, even if guideline developers strictly adhere to the framework, the subjective nature of expert consensus (Yao et al., 2021), potential conflicts of interest (Stahl, 2013; Joshi et al., 2019), and lack of transparency in many guidelines still present severe risks for clinical practice.
Furthermore, this analysis found that each off-label drug recommendation had an average of 4.3 references. A diverse range of literature types was cited, including books (Cheung et al., 2018), databases (Grover and Avasthi, 2019), internet resources (Leboulanger et al., 2020), instructions (Caffarelli et al., 2019), and scientific letters (Lim et al., 2020). There is a research gap in how to grade evidence from these types of sources in the optimal way (AAP, 2004; Atkins et al., 2004b; Milano, 2015; SIGN, 2017; Blanco-Reina et al., 2017). It is also worth noting that 15% of off-label drug recommendations lacked references, being based on either expert opinions (Menter et al., 2020), or even not reporting the source (Litwin et al., 2018).
Reporting the Recommendation on Off-Label Drug Use in Pediatric Guidelines
Off-label drug use is presented in the recommendations in diverse ways. Drug-specific guidelines usually report detailed information on the drug, such as pharmacological effects, pharmacokinetic characteristics, dosage or precise dosage, indications, route of administration, co-administration, regimen, contraindications, precautions, adverse reactions, and monitoring (Simm et al., 2018; Zhou P. et al., 2021). In clinical guidelines not focusing on any specific drug, there is however wide variation in how the recommendations on off-label drug use are reported. Indications and methods of administration tend to be reported frequently, whereas information on the dose or accurate dosage is often lacking, and sometimes even the safety indicators are not appropriately addressed. Given the legal (Mayrhofer, 2014) and safety risks (Schrier et al., 2020) associated with pediatric off-label use of drugs (Schrier et al., 2020), it is also critical to clearly describe if a drug in a recommendation is beyond instructions, since the approval of instructions indicates the availability of high-quality evidence-based support. However, less than 15% of the guidelines for children included in this study specifically noted that the recommended use was off-label.
NSAIDs Used Off-Label
Although many pediatric guidelines suggest NSAIDs or paracetamol for pain, fever or inflammation in children (Turner et al., 2018; Vittinghoff et al., 2018; Mitchell et al., 2019; Ozen et al., 2019; Pirelli et al., 2019; Ringold et al., 2019; Wahezi et al., 2020), they should be taken with caution due to the possibility of adverse reactions such as hypersensitivity reactions (Kidon et al., 2018), renal damage (Gong et al., 2021), and even kidney failure (Gong et al., 2021; Bensman, 2019). However, the reporting of recommendations on NSAIDs in pediatric guidelines is inadequate, which is due to several reasons and may potentially lead to inappropriate drug use. First, pediatric guidelines are usually applicable to children of all ages, but the recommendations do not specify which age groups of children they apply to (Turner et al., 2018). Second, there is a wide range of NSAIDs available, with different therapy ranges and approved ages. Despite this, the recommendations (Ringold et al., 2019) do not declare or clarify which specific NSAIDs are suggested, which might lead to confusion in clinical practice. Finally, as shown in Table 3, the information on NSAIDs stated in the recommendations differs greatly between the guidelines (Turner et al., 2018; Vittinghoff et al., 2018; Ozen et al., 2019; Ringold et al., 2019; Wahezi et al., 2020). There are discrepancies in the content and degree of detail stated in different guidelines when NSAIDs are proposed as off-label drugs, which might be attributable to subjective selection by guideline developers.
Off-Label Drugs Not on World Health Organization Model List of Essential Medicines for Children
In our research, many off-label drugs recommended by guidelines are not on the World Health Organization Model List of Essential Medicines for Children, but they are evidence-based or rational in many cases. WHO has developed a list of essential medicines mainly based on the safety, efficacy, and cost-effectiveness of medicines over the past 44 years (WHO, 2021a), but the committee has not taken into account feedback from professional societies and organizations that develop clinical guidelines, resulting in the exclusion of medicines with better evidence from the list (Dugani et al., 2018). On the other hand, off-label drugs that are not on the list and recommendations that are not supported by the evidence should be noticed and considered, such as infliximab for psoriasis (Menter et al., 2020), which might have a possible conflict of interest. According to our analysis, more than 10% of off-label drug guidelines involve possible commercial promotion, consistent with the current situation (Abbas et al., 2018).
Strengths and Limitations
This study is to our knowledge the first systematic analysis of the reporting of off-label drug recommendations in pediatric guidelines and reveals some gaps in reporting. To ensure a correct extraction of data on off-label drug use, experienced clinical pharmacists reviewed and analyzed the recommendations independently, and inconsistencies were discussed with pediatric clinical experts. However, this study also has some limitations. The study only included English-language guidelines from the last 3 years, without searching websites for additional guidelines. Local web pages or local medical organizations’ web pages usually publish guidelines in non-English, which may include more irrational off-label drug recommendations (Meng et al., 2022). To ensure consistency, the FDA prescription instructions were adopted as criteria for categorizing the type of off-label use of drugs, which may not be completely consistent with the drug instructions used in the target setting of the guideline.
Conclusion
Recommendations on off-label drug use are commonly given in pediatric guidelines, despite the substantial risk of clinical practice being misled. The quality of the evidence supporting the recommendations does not however always match the strength of the recommendations; the recommendations were often based on expert opinion and lacked a declaration of the methodology; and the content and extent of the information reported for recommended off-label drugs varied greatly between the guidelines. Most recommendations involving off-label drug use did not explicitly state that the recommended administration is off-label, which may easily lead to inappropriate off-label use of drugs in children.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
YC and QZ designed the study and edited the article. MM, MT, WL, PW, YL, and YS searched the data. MM, MT, and QL analyzed the data. MM and MT drafted the article. QL and YC revised the article. All authors approved the final version of the article.
Funding
This research was funded by the Chevidence Lab Child & Adolescent Health of Chongqing Medical University’s Children’s Hospital’s Key Project in 2021. The study’s design and execution were not influenced by the major project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge Janne Estill, MD, PhD (University of Geneva), for his assistance in writing the article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.892574/full#supplementary-material
References
Abbas, M., Pires, D., Peters, A., Morel, C. M., Hurst, S., and Holmes, A. (2018). Conflicts of Interest in Infection Prevention and Control Research: No Smoke without Fire. A Narrative Review. Intensive Care Med. 44 (10), 1679–1690. doi:10.1007/s00134-018-5361-z
Agrawal, S., Vamadevan, P., Mazibuko, N., Bannister, R., Swery, R., Wilson, S., et al. (2019). A New Method for Ethical and Efficient Evidence Generation for Off-Label Medication Use in Oncology (A Case Study in Glioblastoma). Front. Pharmacol. 10, 681. doi:10.3389/fphar.2019.00681
Allen, H. C., Garbe, M. C., Lees, J., Aziz, N., Chaaban, H., Miller, J. L., et al. (2018). Off-Label Medication Use in Children, More Common Than We Think: A Systematic Review of the Literature. J. Okla State Med. Assoc. 111 (8), 776–783.
American Academy of Pediatrics Steering Committee on Quality Improvement and Management (2004). Classifying Recommendations for Clinical Practice Guidelines. Pediatrics 114 (3), 874–877. doi:10.1542/peds.2004-1260
Andrews, J. C., Schünemann, H. J., Oxman, A. D., Pottie, K., Meerpohl, J. J., Coello, P. A., et al. (2013). GRADE Guidelines: 15. Going from Evidence to Recommendation-Determinants of a Recommendation's Direction and Strength. J. Clin. Epidemiol. 66 (7), 726–735. doi:10.1016/j.jclinepi.2013.02.003
Aronson, J. K., and Ferner, R. E. (2017). Unlicensed and Off-Label Uses of Medicines: Definitions and Clarification of Terminology. Br. J. Clin. Pharmacol. 83 (12), 2615–2625. doi:10.1111/bcp.13394
Atkins, D., Best, D., Briss, P. A., Eccles, M., Falck-Ytter, Y., Flottorp, S., et al. (2004a). Grading Quality of Evidence and Strength of Recommendations. Bmj 328 (7454), 1490. doi:10.1136/bmj.328.7454.1490
Atkins, D., Eccles, M., Flottorp, S., Guyatt, G. H., Henry, D., Hill, S., et al. (2004b). Systems for Grading the Quality of Evidence and the Strength of Recommendations I: Critical Appraisal of Existing Approaches the GRADE Working Group. BMC Health Serv. Res. 4 (1), 38. doi:10.1186/1472-6963-4-38
Balan, S., Hassali, M. A. A., and Mak, V. S. L. (2018). Two Decades of Off-Label Prescribing in Children: a Literature Review. World J. Pediatr. 14 (6), 528–540. doi:10.1007/s12519-018-0186-y
Bensman, A. (2019). Non-steroidal Anti-inflammatory Drugs (NSAIDs) Systemic Use: The Risk of Renal Failure. Front. Pediatr. 7, 517. doi:10.3389/fped.2019.00517
Blanco-Reina, E., Muñoz-García, A., Cárdenas-Aranzana, M. J., Ocaña-Riola, R., and Del Prado-Llergo, J. R. (2017). Assessment of Off-Label Prescribing: Profile, Evidence and Evolution. Farm Hosp. 41 (4), 458–469. doi:10.7399/fh.2017.41.4.10562
Caffarelli, C., Paravati, F., El Hachem, M., Duse, M., Bergamini, M., Simeone, G., et al. (2019). Management of Chronic Urticaria in Children: a Clinical Guideline. Ital. J. Pediatr. 45 (1), 101. doi:10.1186/s13052-019-0695-x
Carmack, M., Hwang, T., and Bourgeois, F. T. (2020). Pediatric Drug Policies Supporting Safe and Effective Use of Therapeutics in Children: A Systematic Analysis. Health Aff. (Millwood) 39 (10), 1799–1805. doi:10.1377/hlthaff.2020.00198
Casali, P. G., Bruzzi, P., Bogaerts, J., and Blay, J. Y. (2015). Rare Cancers Europe (RCE) Methodological Recommendations for Clinical Studies in Rare Cancers: a European Consensus Position Paper. Ann. Oncol. 26 (2), 300–306. doi:10.1093/annonc/mdu459
Cheng, A. C., Robinson, P. M., and Harvey, K. (2007). Off-label Use of Medicines: Consensus Recommendations for Evaluating Appropriateness. Med. J. Aust. 186 (7), 379–380. doi:10.5694/j.1326-5377.2007.tb00947.x
Cheung, A. H., Zuckerbrot, R. A., Jensen, P. S., Laraque, D., and Stein, R. E. K. (2018). Guidelines for Adolescent Depression in Primary Care (GLAD-PC): Part II. Treatment and Ongoing Management. Pediatrics 141 (3). doi:10.1542/peds.2017-4082
Djulbegovic, B., and Guyatt, G. H. (2017). Progress in Evidence-Based Medicine: a Quarter Century on. Lancet (London, Engl. 390 (10092), 415–423. doi:10.1016/S0140-6736(16)31592-6
Dugani, S., Wasan, K. M., and Kissoon, N. (2018). World Health Organization and Essential Medicines. J. Pharm. Sci. 107 (5), 1261–1262. doi:10.1016/j.xphs.2017.12.019
Eguale, T., Buckeridge, D. L., Verma, A., Winslade, N. E., Benedetti, A., Hanley, J. A., et al. (2016). Association of Off-Label Drug Use and Adverse Drug Events in an Adult Population. JAMA Intern. Med. 176 (1), 55–63. doi:10.1001/jamainternmed.2015.6058
Frattarelli, D. A., Galinkin, J. L., Green, T. P., Johnson, T. D., Neville, K. A., Paul, I. M., et al. (2014). Off-label Use of Drugs in Children. Pediatrics 133 (3), 563–567. doi:10.1542/peds.2013-4060
Gong, J., Ma, L., Li, M., Ma, L., Chen, C., Zhao, S., et al. 31 (2021). Non-steroidal Anti-inflammatory Drugs Associated Acute Kidney Injury in Hospitalized Children: a Systematic Review and Meta-Analysis. Pharmacoepidemiol Drug Saf. doi:10.1002/pds.5385
Grover, S., and Avasthi, A. (2019). Clinical Practice Guidelines for the Management of Schizophrenia in Children and Adolescents. Indian J. Psychiatry 61 (Suppl. 2), 277–293. doi:10.4103/psychiatry.IndianJPsychiatry_556_18
Hudgins, J. D., Bacho, M. A., Olsen, K. L., and Bourgeois, F. T. (2018). Pediatric Drug Information Available at the Time of New Drug Approvals: A Cross-Sectional Analysis. Pharmacoepidemiol Drug Saf. 27 (2), 161–167. doi:10.1002/pds.4351
Hwang, T. J., Tomasi, P. A., and Bourgeois, F. T. (2018). Delays in Completion and Results Reporting of Clinical Trials under the Paediatric Regulation in the European Union: A Cohort Study. PLoS Med. 15 (3), e1002520. doi:10.1371/journal.pmed.1002520
Jiang, Q., Zeng, W., Yu, J., Liu, H., Mao, M., and Li, Y. (2020). Development of the First Value Assessment Index System for Off-Label Use of Antineoplastic Agents in China: A Delphi Study. Front. Pharmacol. 11, 771. doi:10.3389/fphar.2020.00771
Joshi, G. P., Benzon, H. T., Gan, T. J., and Vetter, T. R. (2019). Consistent Definitions of Clinical Practice Guidelines, Consensus Statements, Position Statements, and Practice Alerts. Anesth. analgesia 129 (6), 1767–1770. doi:10.1213/ANE.0000000000004236
Kidon, M., Blanca-Lopez, N., Gomes, E., Terreehorst, I., Tanno, L., Ponvert, C., et al. (2018). EAACI/ENDA Position Paper: Diagnosis and Management of Hypersensitivity Reactions to Non-steroidal Anti-inflammatory Drugs (NSAIDs) in Children and Adolescents. Pediatr. Allergy Immunol. 29 (5), 469–480. doi:10.1111/pai.12915
Kochanek, P. M., Tasker, R. C., Carney, N., Totten, A. M., Adelson, P. D., Selden, N. R., et al. (2019). Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Neurosurgery 84 (6), 1169–1178. doi:10.1093/neuros/nyz051
Lagler, F. B., Hirschfeld, S., and Kindblom, J. M. (2021). Challenges in Clinical Trials for Children and Young People. Arch. Dis. Child. 106 (4), 321–325. doi:10.1136/archdischild-2019-318676
Leboulanger, N., Sagardoy, T., Akkari, M., Ayari-Khalfallah, S., Celerier, C., Fayoux, P., et al. (2020). COVID-19 and ENT Pediatric Otolaryngology during the COVID-19 Pandemic. Guidelines of the French Association of Pediatric Otorhinolaryngology (AFOP) and French Society of Otorhinolaryngology (SFORL). Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 137 (3), 177–181. doi:10.1016/j.anorl.2020.04.010
Li, Y., Jia, L., and Teng, L. (2016). A Systematic Review of Off-Label Drug Use at Home and Abroad for Pediatrics. Chin. J. Hosp. Pharm. 36 (23), 2114–2119.
Lim, J. S., Kim, E. Y., Kim, J. H., Yoo, J. H., Yi, K. H., Chae, H. W., et al. (2020). Clinical Practice Guidelines for Dyslipidemia of Korean Children and Adolescents. Ann. Pediatr. Endocrinol. Metab. 25 (4), 199–207. doi:10.6065/apem.2040198.09910.3345/cep.2020.01340
Litwin, M., Niemirska, A., Obrycki, Ł., Myśliwiec, M., Szadkowska, A., Szalecki, M., et al. (2018). Guidelines of the Pediatric Section of the Polish Society of Hypertension on Diagnosis and Treatment of Arterial Hypertension in Children and Adolescents. 22(2), 45–73. doi: doi:10.5603/ah.2018.0007
Liu, E., Smyth, R. L., Luo, Z., Qaseem, A., Mathew, J. L., Lu, Q., et al. (2020). Rapid Advice Guidelines for Management of Children with COVID-19. Ann. Transl. Med. 8 (10), 617. doi:10.21037/atm-20-3754
Mayrhofer, M. (2014). Off Label Use of Analgesics in Pediatric Perioperative Pain Therapy from a Legal Perspective: Austrian Interdisciplinary Recommendations on Pediatric Perioperative Pain Management. Schmerz 28 (1), 65–66. doi:10.1007/s00482-013-1385-z
Meng, M., Wang, P., Lan, H., Lei, W., Shen, Q., Zhou, Q., et al. (2022). Analysis on Off-Label Use of Drugs in Pediatric Guidelines and Consensus Published by Chinese Authors. Chin. J. Pediatr. 60 (3), 215–220. doi:10.3760/cma.j.cn112140-20210923-00813
Menter, A., Cordoro, K. M., Davis, D. M. R., Kroshinsky, D., Paller, A. S., Armstrong, A. W., et al. (2020). Joint American Academy of Dermatology-National Psoriasis Foundation Guidelines of Care for the Management and Treatment of Psoriasis in Pediatric Patients. J. Am. Acad. Dermatol 82 (1), 161–201. doi:10.1016/j.jaad.2019.08.049
Milano, G. (2015). The Hierarchy of the Evidence-Based Medicine Pyramid: Classification beyond Ranking. Joints 3 (3), 101. doi:10.11138/jts/2015.3.3.101
Mitchell, R. B., Archer, S. M., Ishman, S. L., Rosenfeld, R. M., Coles, S., Finestone, S. A., et al. (2019). Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol. Head. Neck Surg. 160 (1_Suppl. l), S1–s42. doi:10.1177/019459981880175710.1177/0194599818807917
Ozen, S., Marks, S. D., Brogan, P., Groot, N., de Graeff, N., Avcin, T., et al. (2019). European Consensus-Based Recommendations for Diagnosis and Treatment of Immunoglobulin A Vasculitis-The SHARE Initiative. Rheumatol. Oxf. 58 (9), 1607–1616. doi:10.1093/rheumatology/kez041
Pirelli, A., Savant Levet, P., Garetti, E., Ancora, G., Merazzi, D., Bellieni, C. V., et al. (2019). Literature Review Informs Clinical Guidelines for Pain Management during Screening and Laser Photocoagulation for Retinopathy of Prematurity. Acta Paediatr. 108 (4), 593–599. doi:10.1111/apa.14523
Pocai, B. (2019). The ICD-11 Has Been Adopted by the World Health Assembly. World Psychiatry 18 (3), 371–372. doi:10.1002/wps.20689
Remi, C., Weingärtner, K., Hagemann, V., Bausewein, C., and Hodiamont, F. (2021). Off-label Drugs in Palliative Care: a Group Delphi Treatment Recommendation Process. BMJ Support Palliat. Care 11 (2), 180–187. doi:10.1136/bmjspcare-2019-002165
Ringold, S., Angeles-Han, S. T., Beukelman, T., Lovell, D., Cuello, C. A., Becker, M. L., et al. (2019). 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. Hob. 71 (6), 717–734. doi:10.1002/acr.2387010.1002/art.40884
Rosen, R., Vandenplas, Y., Singendonk, M., Cabana, M., DiLorenzo, C., Gottrand, F., et al. (2018). Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 66 (3), 516–554. doi:10.1097/mpg.0000000000001889
Schrier, L., Hadjipanayis, A., Stiris, T., Ross-Russell, R. I., Valiulis, A., Turner, M. A., et al. (2020). Off-label Use of Medicines in Neonates, Infants, Children, and Adolescents: a Joint Policy Statement by the European Academy of Paediatrics and the European Society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 179 (5), 839–847. doi:10.1007/s00431-019-03556-9
Scottish Intercollegiate Guidelines Network (2017). Key to Evidence Statements and Grades of Recommendations. Management.
Sharma, A. N., Arango, C., Coghill, D., Gringras, P., Nutt, D. J., Pratt, P., et al. (2016). BAP Position Statement: Off-Label Prescribing of Psychotropic Medication to Children and Adolescents. J. Psychopharmacol. 30 (5), 416–421. doi:10.1177/0269881116636107
Simm, P. J., Biggin, A., Zacharin, M. R., Rodda, C. P., Tham, E., Siafarikas, A., et al. (2018). Consensus Guidelines on the Use of Bisphosphonate Therapy in Children and Adolescents. J. Paediatr. Child. Health 54 (3), 223–233. doi:10.1111/jpc.13768
Slater, R., Moultrie, F., Bax, R., van den Anker, J., and Bhatt, A. (2020). Preterm Health: Time to Bridge the Evidence Gap. Lancet 396 (10255), 872–873. doi:10.1016/s0140-6736(20)31977-2
Stahl, S. M. (2013). Off-label Prescribing: Best Practice or Malpractice? CNS Spectr. 18 (1), 1–4. doi:10.1017/s1092852913000011
Stolbach, A. I., Mazer-Amirshahi, M., Marino, R., Nelson, L. S., and Sugarman, J. (2020). ACMT Position Statement: Off-Label Prescribing during COVID-19 Pandemic. J. Med. Toxicol. 16 (3), 342–345. doi:10.1007/s13181-020-00784-6
Sweileh, W. M. (2021). 24 Global Research Publications on Systemic Use of Off-Label and Unlicensed Drugs: A Bibliometric Analysis (1990-2020). Int. J. Risk Saf. Med. doi:10.3233/jrs-210012
The World Bank (2022). Countries and Economies [Online]. Available at: https://data.worldbank.org/country.
Tobin, J. R. (2010). Use of Pharmaceuticals 'off-Label' in the Neonate. Best. Pract. Res. Clin. Anaesthesiol. 24 (3), 451–460. doi:10.1016/j.bpa.2010.02.015
Turner, D., Ruemmele, F. M., Orlanski-Meyer, E., Griffiths, A. M., de Carpi, J. M., Bronsky, J., et al. (2018). Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-Based Consensus Guideline from the European Crohn's and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 67 (2), 292–310. doi:10.1097/mpg.0000000000002036
Ucosfdtcp, I. o. m. (2011). Clinical Practice Guidelines We Can Trust. Washington (DC): National Academies Press US.
Vittinghoff, M., Lönnqvist, P. A., Mossetti, V., Heschl, S., Simic, D., Colovic, V., et al. (2018). Postoperative Pain Management in Children: Guidance from the Pain Committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr. Anaesth. 28 (6), 493–506. doi:10.1111/pan.13373
Wahezi, D. M., Lo, M. S., Rubinstein, T. B., Ringold, S., Ardoin, S. P., Downes, K. J., et al. (2020). American College of Rheumatology Guidance for the Management of Pediatric Rheumatic Disease during the COVID-19 Pandemic: Version 1. Arthritis Rheumatol. 72 (11), 1809–1819. doi:10.1002/art.41455
Whitley, L. E., and Chepke, C. (2020). A Pediatric Neurobehavioral Treatment Challenge. eNeurologicalSci 20, 100244. doi:10.1016/j.ensci.2020.100244
WHO (2021a). Executive Summary: The Selection and Use of Essential Medicines 2021: Report of the 23rd WHO Expert Committee on the Selection and Use of Essential Medicines. [Online]. Available at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.(Accessed 01 2021).
WHO (2021b). WHO Model List of Essential Medicines for Children. [Online]. Available at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.03. (Accessed 01 2021).
Wu, W., Tang, Z., Chen, J., and Gao, Y. (2019). Pediatric Drug Development in China: Reforms and Challenges. Pharmacol. Res. 148, 104412. doi:10.1016/j.phrs.2019.104412
Yao, L., Ahmed, M. M., Guyatt, G. H., Yan, P., Hui, X., Wang, Q., et al. (2021). Discordant and Inappropriate Discordant Recommendations in Consensus and Evidence Based Guidelines: Empirical Analysis. Bmj 375, e066045. doi:10.1136/bmj-2021-066045
Zhou, P., Wang, X., Zhang, X., Xu, B., Tong, X., Zhou, W., et al. (2021a). Recommendations on Off-Label Use of Intravenous Azithromycin in Children. Int. J. Clin. Pract. 75 (7), e14010. doi:10.1111/ijcp.14010
Keywords: pediatric guidelines, recommendations, off-label use of drugs, evidence-based medicine, children
Citation: Meng M, Zhou Q, Lei W, Tian M, Wang P, Liu Y, Sun Y, Chen Y and Li Q (2022) Recommendations on Off-Label Drug Use in Pediatric Guidelines. Front. Pharmacol. 13:892574. doi: 10.3389/fphar.2022.892574
Received: 09 March 2022; Accepted: 20 April 2022;
Published: 09 June 2022.
Edited by:
Luciane Cruz Lopes, University of Sorocaba, BrazilReviewed by:
Albert Figueras, Universitat Autònoma de Barcelona, SpainJoel Lexchin, York University, Canada
Isabela Heineck, Federal University of Rio Grande do Sul, Brazil
Copyright © 2022 Meng, Zhou, Lei, Tian, Wang, Liu, Sun, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Li, bGlxaXU4MDlAaG9zcGl0YWwuY3FtdS5lZHUuY24=; Yaolong Chen, Y2hldmlkZW5jZUBsenUuZWR1LmNu
 Min Meng1,2,3,4
Min Meng1,2,3,4 Yunlan Liu
Yunlan Liu Yajia Sun
Yajia Sun Yaolong Chen
Yaolong Chen Qiu Li
Qiu Li