
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 July 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.908487
This article is part of the Research Topic Phytochemicals in Cancer Prevention: Recent Trends and Advances View all 6 articles
 Tomi Lois Adetunji1
Tomi Lois Adetunji1 Femi Olawale2
Femi Olawale2 Chijioke Olisah3
Chijioke Olisah3 Ademola Emmanuel Adetunji4*
Ademola Emmanuel Adetunji4* Adeyemi Oladapo Aremu5,6*
Adeyemi Oladapo Aremu5,6*Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is one of the most important natural products in the genus Capsicum. Due to its numerous biological effects, there has been extensive and increasing research interest in capsaicin, resulting in increased scientific publications in recent years. Therefore, an in-depth bibliometric analysis of published literature on capsaicin from 2001 to 2021 was performed to assess the global research status, thematic and emerging areas, and potential insights into future research. Furthermore, recent research advances of capsaicin and its combination therapy on human cancer as well as their potential mechanisms of action were described. In the last two decades, research outputs on capsaicin have increased by an estimated 18% per year and were dominated by research articles at 93% of the 3753 assessed literature. In addition, anti-cancer/pharmacokinetics, cytotoxicity, in vivo neurological and pain research studies were the keyword clusters generated and designated as thematic domains for capsaicin research. It was evident that the United States, China, and Japan accounted for about 42% of 3753 publications that met the inclusion criteria. Also, visibly dominant collaboration nodes and networks with most of the other identified countries were established. Assessment of the eligible literature revealed that the potential of capsaicin for mitigating cancer mainly entailed its chemo-preventive effects, which were often linked to its ability to exert multi-biological effects such as anti-mutagenic, antioxidant and anti-inflammatory activities. However, clinical studies were limited, which may be related to some of the inherent challenges associated with capsaicin in the limited clinical trials. This review presents a novel approach to visualizing information about capsaicin research and a comprehensive perspective on the therapeutic significance and applications of capsaicin in the treatment of human cancer.
Capsaicin (8-Methyl-N-vanillyl-trans-6-nonenamide, C18H27NO3) is a homovanillic acid abundant in Capsicum species (pepper) fruits (1, 2). Its structure (Supplementary Figure 1) was ascertained in 1919, following its isolation in 1846 (3, 4) and chemical synthesis in 1930 (5). The compound has two geometric (cis-trans) isomers but naturally occurs as a trans-isomer (6). This lipophilic compound is the main bioactive constituent of peppers and is responsible for the tissue irritation and characteristic burning consequences (pungency) of peppers (1, 2). It accounts for about 70% of the alkaloid group called capsaicinoids. Other analogs are dihydrocapsaicin (second most abundant, representing ca. 22%), nordihydrocapsaicin (ca. 7%), and compounds such as homocapsaicin, homodihydrocapsaicin and norcapsaicin produced in lower quantities (2). Capsaicin constitutes a key ingredient of self-protection products (e.g., oleoresin capsicum spray), spicy foods in various cultures around the world, and its concentration may be more than 65% in cosmetic, herbal supplements, and other health care products (7).
Capsaicin content is high in pepper fruit placenta, which holds the seeds (3, 8). The ovary and fruit–tip contain the highest capsaicin content, while the seeds have the lowest concentration (9). Capsaicin levels may increase in pepper when subjected to controlled-stress conditions (10, 11). Due to its broad applicability, there have been extensive studies aimed at enhancing capsaicin production. For instance, capsaicin production has been improved by enzyme-catalyzed (12), chemical (13), and in vitro syntheses (14, 15) as well as improving pepper cultivation (6, 16). Its biosynthesis by fatty acid metabolism and phenylpropanoid pathways (Supplementary Figure 2) has been described by several authors (10, 11, 17).
There is increasing interest in using capsaicin as a therapeutic alternative for different diseases (18, 19) due to its pleiotropic pharmacological effects on various physiological systems, with an emphasis on pain as well as neuroscience, cardiovascular, respiratory, cancer, and urinary systems studies (20). In terms of the pharmacokinetics, capsaicin has high oral bioavailability and skin absorption (21), making its topical application effective in various musculoskeletal or neuropathic pain conditions such as arthritis (22), shingles (23), vasomotor rhinitis (24), vasogenic facial pain (25). It is also used for treating urinary incontinence, chronic kidney disease-associated pruritus, and postoperative nausea and vomiting in acupoint therapy (3). Other beneficial bioactivities of capsaicin, including analgesic, anesthetic, anti-apoptotic, anti-inflammatory, anti-obesity, antioxidant, neuroprotective effects (1, 26, 27), enhanced energy metabolism (28), gastroprotective (29), and anticarcinogenic properties (7, 30) have also been reported. However, capsaicin may also function as a carcinogen or co-carcinogen (7, 31, 32).
Understanding capsaicin research from global perspectives over an extended time is crucial. Although several studies have been published on capsaicin applications, bioactivities, and many other capsaicin-related topics (33–35), none of these studies explored the scientometric approach to critically assess its progress and current direction in scientific research. Bibliometrics is a valuable tool for evaluating research trends within a subject area, thus providing insight into extensively researched themes and identifying research needs to inform action (36).
Web of Science Core Collection indexing coverage search gave more than 19,000 publications about capsaicin from 1991 to 2021. A recent review also showed that out of over 10,000 capsaicin-related publications from 2010 to 2020, the anticancer effect was the most investigated, accounting for ca. 26% (2). Hence, this review aimed to provide a systematic review of global research output and recent advances in capsaicin application against human cancer in the last two decades. The in-depth analysis of retrieved publications provided an overview of explored themes, research progression, as well as insights and future perspectives needed to enrich the knowledge domain on the compound.
Data used for the bibliometric survey was retrieved according to the procedure described in our previous articles (36, 37)(Supplementary Figure 3). Concisely, published articles on “Capsaicin” were retrieved from the Web of Science (WoS) Core Collection and Scopus databases. The former database was chosen because it contains a high volume of biological and physical sciences literature (38, 39), while Scopus is considered the largest citation and abstract source of global research outputs (40). In the WoS database, the search term “Capsaicin” was used to retrieve records in the “Title” module from January 1, 2001 to December 31, 2021. Only document types such as “Article”, “Review”, “Book Chapter”, and “Editorial” were searched. The search yielded 2914 records. Other document types such as “Proceeding Paper,” “Letters,” “News Items,” “Corrections,” “Early Access,” “Retracted Publications,” and “Publication with Expression of Concern,” were excluded from the search because these are often pre- or post-publication data. For Scopus, a total of 3261 records were identified using the search term “Capsaicin” on the “Article Title” search field. Only records such as “Article”, “Review”, “Editorial”, and “Book Chapter” that satisfied the selection criteria were included. Other records such as “Conference Paper,” “Note,” “Letter,” “Erratum,” “Short Survey,” and “Retracted” were excluded. Records from both databases were downloaded in Bibtex file format and uploaded in RStudio (Version 1.1.463, 2009–2018) for statistical processing. The search and data retrieval were conducted on January 31, 2022. Bibliometric library and packages were installed on the RStudio and used to analyse all bibliometric indicators (articles produced per year, most used keywords, most productive authors, and countries based on number of publications and citations). Duplicate records from both databases were merged as one using R commands. Codes for all bibliometric indicators were obtained from https://www.bibliometrix.org/vignettes/Introductiontobibliometrix.html. Keyword visualization was done on VOSviewer (version 1.6.15, 2009 –2020). Information on recent research advances of capsaicin against human cancer was obtained from relevant articles published in the last two decades in different databases, including Google Scholar, WoS, and Scopus. Chemical structures were drawn with ChemDraw.
A total of 3753 scientific documents met the inclusion criteria in the merged databases (WoS and Scopus) across the 20 years from 2001 to 2021. The entire capsaicin-related research was divided into five groups according to the type of document: articles (3460) representing 93% of the total, followed by reviews (195, 5%), editorial materials (43, 1.2%), book chapters (54, 1.4%), and books (1). Documents were retrieved from 1385 journals and included 6920 author keywords (DE) and 12586 keywords plus (ID). Except for 115 single-authored publications, all authors (10113) published multiple-author articles with an average author-per-document and document-per-author of 2.69 and 0.371, respectively (Supplementary Table 1).
Figure 1 presents the annual scientific production and trends in capsaicin research from 2001 to 2021. Given its wide display of biological effects and therapeutic significance since its identification, capsaicin has been the target of extensive research (41). The number of articles related to capsaicin research increased from 162 in 2001 to 239 in 2021, with an annual growth rate of 18.88%. From 2001 to 2017, it was observed that there were fluctuations in publication productivity. However, in 2018, the publication productivity of capsaicin research increased steadily up until 2021. The relationship between the publication year and the number of publications fitted into the polynomial model showed a strong positive correlation r2 value of 0.956. This result, together with other statistical measures, such as Kolmogorov-Smirnoff goodness-of-fit (0.719) and β-coefficient (2. 446), suggests that there could be an increasing trend in publication productivity with persistent investigations.
Regarding the publication language, the majority of the articles were published in English (98%), however, some articles were also published in German (0.5%), French (0.3%), and Spanish (0.2%). Other languages, such as Chinese, Portuguese, Turkish and Polish, occurred in lower frequencies. Evidently, capsaicin research topics are rising trends in scientific research, and several researchers across the globe are actively contributing to the field. The diversity of research topics on capsaicin is evident in the distribution of different publications in science-based subject areas, including neurosciences, pharmacology, biochemistry and/or molecular biology, physiology, chemistry, anaesthesiology, cell biology, food science and technology, science technology and gastroenterology (hepatology). This underscores the recognition of capsaicin as a promising drug candidate to be developed as a primary treatment therapy for several ailments (41).
In scientometric analysis, keywords in publications are generally accepted as representations for obtaining insights into the thematic area of the research (42). Here, the top 20 most relevant keywords [author’s keywords (DE) and keyword-plus (ID)] in capsaicin research were recorded (Supplementary Table 2). To evaluate the thematic areas of capsaicin-related publications, an analysis of the co-occurrence network of keywords associated with capsaicin research was done for the period under study. Four keyword clusters can be interpreted as the thematic areas in the study domain, where each cluster represents a thematic domain (Figure 2). The terms enclosed in different coloured circles in a cluster represent the most frequently used keywords. Lines between terms show the frequency of occurrence in literature.
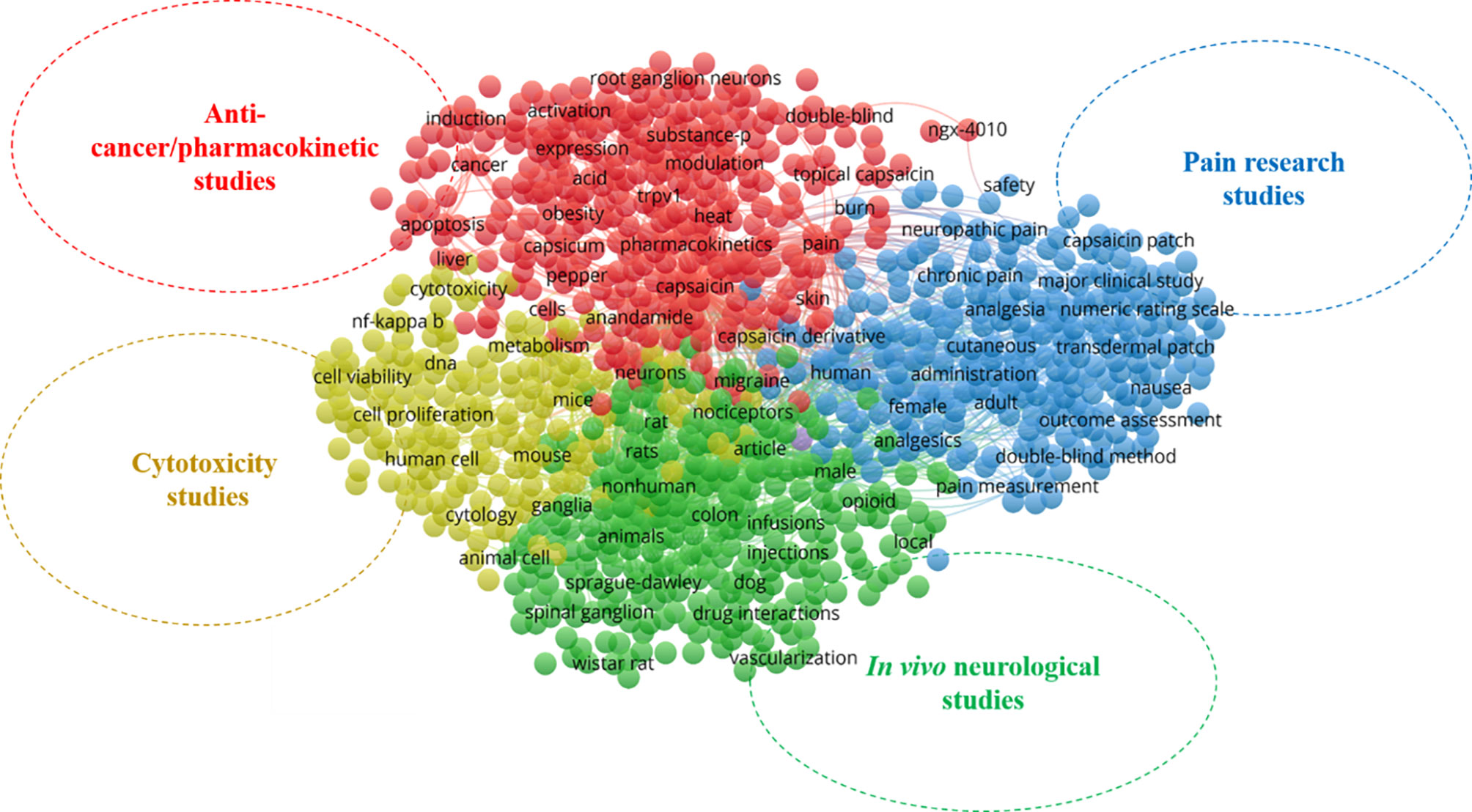
Figure 2 Thematic areas and network visualization of keyword co-occurrence map on capsaicin publications.
In Figure 2, the blue cluster (cluster 1) represents the first thematic area. It focuses on pain research studies on capsaicin with different keywords such as pain measurement, analgesics, neuropathic pain, and chronic pain. Indeed, capsaicin is an incontestably thrilling molecule and remains a valued drug for easing pain (41). Currently, capsaicin is in the third phase (phase III) of clinical trials as an analgesic agent for musculoskeletal, chronic/acute, arthritis, neuropathic, and postoperative pains (41, 43).
The green cluster (cluster 2), with keywords such as animals, rats, dogs, Wistar rat, Sprague- Dawley, neurons, nociceptors, spinal ganglions, and ganglia represents in vivo neurological thematic area. This cluster covers publications that document the application of in vivo models to study the mechanism by which capsaicin exerts its many therapeutic effects on the human nervous system. The utilization of capsaicin as a therapeutic agent stems from its relatively selective capacity to excite and or cause neuroinhibitory action (capsaicin desensitization) of a subpopulation of afferent neurons [transient receptor potential channel vanilloid (TRPV1)] receptors, which reduces the number of nerve fibers that respond to painful stimuli (44, 45). Hence, extensive studies have been done in this thematic area over the last two decades.
In the yellow cluster (cluster 3), keywords such as cytotoxicity, human cells, cell viability, cytology, and animal cell focusing on cytotoxicity study thematic area are grouped. Although, with proper dosage, capsaicin has demonstrated several health-promoting effects, high doses of capsaicin can cause various acute physiological responses (e.g., burning sensation), activate inflammation, and induce cytotoxicity in various cells (46). Thus, there has been significant research on the cell toxicity of capsaicin to ensure the most effective dosage in treating different ailments. Apart from the side effects of capsaicin prompting cytotoxicity studies, capsaicin has shown wide applicability against several types of cancer (30) through the induction of apoptosis and arrest of cell cycle progression (47). Hence, studies to explore the effects of capsaicin on cancer cell lines have persisted.
The red cluster (cluster 4) depicts the pharmacokinetics and anticancer thematic area of capsaicin research and includes different keywords such as modulation, expression, induction, activation, TRPV1, cancer and apoptosis. Over the last two decades, there have been several publications investigating the anticancer, mechanisms of action, pharmacokinetics, and pharmacodynamics of capsaicin since this compound exerts many pathways in its mode of action against different ailments.
Research outputs on capsaicin were published in 1385 primary reference works such as conference proceedings, journals, books, and letters. The top 20 most productive journals in capsaicin-related research are recorded in Supplementary Table 3. Based on the compiled data, three publishers − Elsevier, Wiley Blackwell, and Lippincott Williams & Wilkins were identified as the top publishers, with seven Elsevier journals, two Wiley Blackwell journals, and two Lippincott Williams & Wilkins journals. Different publishers publish the remaining journals across the globe. The most productive journal in terms of the number of articles was “Pain” with 363 articles, followed by “European Journal of Pharmacology” (78 articles), “Neuroscience Letters” (63 articles), “Neuroscience” (56 articles), and “Brain Research” (55 articles). Pain, which had the highest number of articles on capsaicin research, is one of the top journals devoted to publishing original research articles that deal with the nature, mechanisms, and treatment of pain. One of the major therapeutic applications of capsaicin is its topical use in treating pain. Capsaicin studied in several in vitro and in vivo models as well as clinical trials has shown therapeutic effectiveness against acute and chronic pains and has been approved as a topical treatment of neuropathic pain (48). The mechanism of action of its pain-relieving effect has been attributed to the ability of capsaicin to cause reversible defunctionalization or desensitization of sensory nerve endings of substance P and by reducing the density of epidermal nerve fibers (49). The increasing interest of researchers in understanding capsaicin’s effects and the mechanisms of action on chronic pain may account for the high number of articles on capsaicin published in Pain journal. In terms of impact factor, the British Journal of Pharmacology (IF 8.739) was the most influential, followed by Pain (IF 6.96), Journal of Neuroscience (IF 6.167), Journal of Pain (IF 5.820), and Journal of Agricultural and Food Chemistry (IF 5.279).
An important aspect of bibliometrics is the contribution of authors toward a research topic. Citation indicators or metrics, especially the H-index (an author-level metric that measures both the productivity and citation impact of the publications), are generally being used in the context of research evaluation (50). Several studies have shown that the H-index is correlated with the total number of citations and publications (51, 52). The present study used different citation metrics such as the number of articles, H-index, g-index, and total citations to identify the top 20 leading authors (authors who have contributed more than 20 publications) in capsaicin research over the last two decades (Supplementary Table 4). The top leading author’s productivity is shown in Supplementary Figure 4, where embedded circles represent the total number of articles and total citations for articles published in a particular year.
The results of the analysis show that the top five most productive authors are Lee, J. (46 articles), Lee, S. (45 articles), Wang, X (43 articles), Wang, Y. (43 articles) and Zhang, Y. (42 articles). As for the relative impact of the publication in terms of citations, Anand, P. (3166 citations), Lee, S. (1763 citations), Wang, X. (1282 citations), Wang, J. (1124 citations) and Wang, H. (1075 citations) were the most influential in the period considered.
The number of citations an article receives has been employed as a marker of its influence on the research community in that subject area (53). We identified the 20 most cited articles in the field of capsaicin research during the 20 years study period (Supplementary Table 5) and their association with the clusters identified in Figure 2 (the thematic areas of capsaicin research). The listed 20 most-cited articles gave insight into the important articles and thematic areas that had impacted capsaicin research within and beyond the subject area. These articles were all published by authors from developed countries and were co-authored collaborations except Amadesi (54) and Ghilardi (55). These top-cited articles received between 240 and 1048 citations, and only four articles were cited more than 400 times. The most cited article was “Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition” by Chuang et al. (56), published in Nature with 1048 citations. By evaluating the listed articles (Supplementary Table 5) in relation to the thematic clusters in Figure 2, it appears that research focusing on the pharmacokinetics/pharmacodynamics of capsaicin (cluster 4) has made the greatest contributions, as six of the 20 most cited publications are associated with this cluster.
The heat map of the 10 top leading countries that have contributed more than 30 publications over the 20 years study period was shown in Figure 3. Additional information given in Supplementary Table 6, showed the number of articles, citations, average article citations, single country publications, and multiple country publications. The highest publication metrics were from the United States (689 publications), China (546 publications), Japan (354 publications), North Korea and South Korea (209 publications), and India (172 publications), making them the top five leading countries in terms of the number of publications. Of the top leading 20 countries, the dominance of European countries and, to a lesser degree, Asian countries was striking, while publications originating from North America, South America, and Oceania were less prevalent. Within Asia, the majority of the publications originated from the far East, and China, Japan, North and South Korea, and India were recognized as significant contributors to capsaicin research. In Europe, Germany, United Kingdom, and Italy were significant contributors to capsaicin publications. Europe has been recognized as the centre of global science and research since the beginning of the 20th century as scientists from relatively rich European countries are heavily funded (57). This could account for the relatively high contributions of capsaicin publications from Europe. In terms of citations, the top three countries — the US (28869 total citations), China (8621 total citations), and Japan (8578 total citations) corresponded with the top three productive countries. Other countries with relatively high citations were the United Kingdom (7857 total citations) and North and South Korea (5813 total citations).
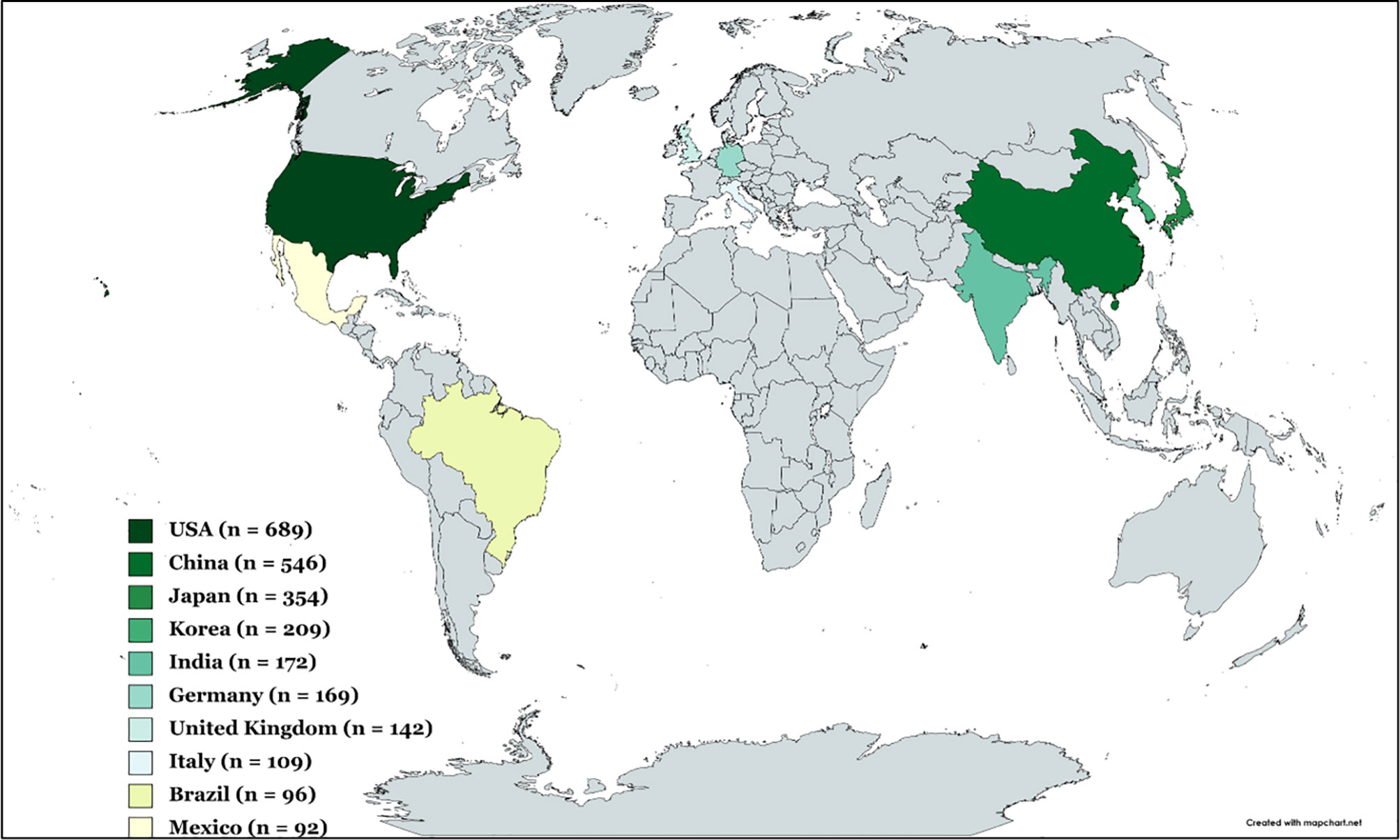
Figure 3 Heat map of the top 10 leading countries based on capsaicin publications from 2001 to 2021. Grey colour shadings signify countries outside the top 10.
To assess the international collaboration network on capsaicin research among the 20 leading countries, a network visualization map was constructed (Supplementary Figure 5). Generally, more productive countries (in terms of publications and citations) also have more collaboration links. The line thickness in the network map depicts the collaboration frequency between countries. The United States had a visibly dominant collaboration node with a strong collaboration network with almost all other countries on the map. China, which had the second-largest node and the second-highest number of publications, collaborated mainly with the US, Denmark, Australia, and Canada.
Non-communicable diseases, including cancer, contribute significantly to morbidity and account for over 70% of untimely global mortality. The vast plurality of these deaths (over 80%) occurs in nations with a low or medium Human Development Index (58). Cancer is considered an increasing public health concern with devastating economic implications (59). With the aging and growing population, about 29 million cancer cases are expected by 2040 (60). Cancer treatment is currently a topic of high interest due to its severity, impact on quality of life, and burden on the healthcare system (61). Despite the advancement of medical science, the burden of cancer keeps rising rapidly and demands safe and more effective cancer prevention and treatment strategies capable of inhibiting or reversing cancer (32). The failure of most of the current treatment strategies has been linked to the fact that different forms of cancer are capable of acquiring mutations that make them resistant to treatment over time.
Lately, however, there has been growing attention to the potential of natural products, including dietary phytochemicals such as capsaicin, as safe and effective therapies for combating cancer (62, 63). This current research paradigm stems from the role of diet in 30% of cancers and the proposition that 35% of cancer can be prevented by diet and lifestyle changes (64, 65). Indeed, dietary phytochemicals have shown significant efficacy in ameliorating several levels of cancer development. Equally advantageous is the fact that these dietary phytoconstituents are readily available, relatively non-toxic, biocompatible, and cheap.
About 25% of global therapeutic drugs have been sourced primarily or otherwise from plants (66). For instance, anticancer agents such as vinblastine and paclitaxel were derived from Catharanthus roseus (L.) G. Don (syn. Vinca rosea L.) and Taxus brevifolia Nutt., respectively (62). Thus, natural therapeutic agents are historically key contributors to drug discovery for various diseases, including cancer (67). Persuasive evidence from experimental and epidemiological studies has shown that some of these plant-derived therapeutic agents possess promising chemopreventive and chemotherapeutic properties (64). Capsaicin, an homovanillic acid derivative, is one of such dietary phytochemicals with the ability to ameliorate cancer at various levels. Various remedial effects and mechanisms of actions of capsaicin have been documented (2, 32, 61). This section considers various anticancer roles of capsaicin and the underlying mechanism in a more detailed perspective.
Chemoprevention refers to the use of chemotherapeutic agents that hinders and halts the development of tumor before the onset of tumor cell invasion (68). Capsaicin has shown significant prospects as an effective chemopreventive agent, as discussed below.
Arguably, the initial evidence of the anticancer role of capsaicin could be traced to earlier studies on the chemopreventive/anticarcinogenic activity. Capsaicin pre-treatment could suppress DNA binding of benzo (a) pyrene (a carcinogenic polycyclic aromatic hydrocarbon), thereby inhibiting lung carcinogenesis in mouse model (69). Several studies further corroborated this research finding with capsaicin showing protective effects against chemical carcinogens such as aflatoxin B1, 4-(methylnitrosamino)- 1-(3-pyridyl)- 1-butanone, vinyl carbamate and N-nitrosodimethylamine (70). It is noteworthy that a number of these hydrocarbons (specifically halogenated hydrocarbons) are metabolized by the phase drug-metabolizing enzyme CYP450 2E1, which catalyzes the activation to generate highly reactive genotoxic products. Interestingly, capsaicin has been found to inhibit several isoforms of CYP 450 enzymes, including CYP 2E1. As such, the chemoprotective role of capsaicin has been linked to its ability to modulate CYP enzymes (7). Furthermore, in a more recent study, capsaicin was shown to cause upstream activation of Ca2+/calmodulin (CaM)-dependent protein kinase (CaMK) and CCAAT/enhancer-binding protein β (C/EBPβ), which resulted in a concomitant inhibition of CYP1A1 mRNA (71). Hence, by inhibiting CYP enzymes expression and its upstream modulator, capsaicin is capable of acting as an anticarcinogenic agent.
Another plausible mechanism implicated in the chemopreventive action of capsaicin is its anti-oxidative effects. Capsaicin elicits a biphasic anticancer action, acting directly to scavenge free radicals and upregulating the expression of several antioxidant enzymes. Antiradical activity of pure capsaicin revealed high scavenging activity against 2,2′-azino-bis(3- ethylbenzothiazoline-6-sulphonic acid (ABTS) radical with IC50 value of 187.7 µM (72). Likewise, there was a positive correlation between the levels of capsaicin and its analogues and the antioxidant activity of peppers of the genus Capsicum (73). Capsaicin was also shown to protect against autoxidation and Fe2+ induced oxidation of linoleic acid (74). In addition, capsaicin inhibits reactive oxygen species (ROS) release and the subsequent mitochondrial membrane potential collapse, cytochrome c expression, chromosome condensation, and caspase-3 activation induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells (75). This profound free radical scavenging activity gives credence to the ability of the compound to mitigate oxidative stress conditions, which have been implicated in cellular dysfunction vis-a-vis the development of cancer.
Aside from directly scavenging free radicals in vitro, capsaicin has also been found to increase the expression of antioxidant enzymes in vivo to modulate oxidative imbalance. Capsaicin pre-treatment in mice suppressed oxidative damage in mice testicles exposed to heat stress by modulating heat shock 70-kDa protein 1 (Hsp72), phospholipid hydroperoxide glutathione peroxidase (PGHPx), and manganese superoxide dismutase (MnSOD) mRNA expression (76). Hsp72 gene is upregulated in response to oxidative stress; however, the pre-exposure to capsaicin results in decreased Hsp72 levels (76). Likewise, the increased expression of MnSOD and PGHPx underscores the role of capsaicin in activating antioxidant enzyme expressions. A similar protective effect was demonstrated in cisplatin-induced nephrotoxicity in rats, where exposure to capsaicin decreased the levels of kidney malondialdehyde and ameliorate decreased levels of GSH and SOD activity (77).
Additionally, capsaicin can act synergistically with other dietary phytochemicals causing an exponential beneficial cytoprotective effect (78, 79). For instance, dietary curcumin and capsaicin concurrent administration in high-fat diet-fed rats were shown to mitigate the testicular and hepatic antioxidant status by increasing GSH levels, glutathione transferase activity, and Cu-ZnSOD expression (79). The investigations by Joung et al. (80) provided further mechanistic insights into the antioxidant defense mechanism of capsaicin. The authors noted that capsaicin was capable of inducing a series of protein kinase phosphorylation events activating the antioxidant defense response in HepG2 cells. Capsaicin was shown to trigger the phosphorylation of Akt, activating the protein kinase leading to Nrf2 phosphorylation (80). The phosphorylation of Nrf2 results in disruption of NRF2/Keap 1 complex liberating the activated Nrf2 protein, which translocates to the nucleus forming a complex with maf2, which binds to the antioxidant response element in the promoter region of genes encoding the antioxidant enzyme heme-oxygenase-1 (Figure 4). HO-1 catalyzes the oxidative degradation of heme to liberate free heme, carbon monoxide and biliverdin. By degrading heme, HO-1 prevents oxidative damage by heme protein. Besides HO-1, Nrf2 activation have also been linked to the increased expression of other and drug-metabolizing enzymes such as NAD(P)H:quinone acceptor oxidoreductase (NQO) and antioxidant enzymes, including catalase (CAT), SOD, GPX and GST via the Nrf2/ARE pathway (81).
Anti-inflammation is another mechanism implicated in the chemopreventive action of capsaicin. According to the national cancer institute, chronic inflammation has been named as a major risk factor in cancer. This is because, during chronic inflammation, notable damage to the DNA structure is observed, which can ultimately result in cancer. Such is the case observed during chronic inflammatory bowel disorders such as Crohn’s disease and ulcerative colitis, which leads to colon cancer. Sub-plantar injections of capsaicin were able to significantly inhibit paw-swelling in Wistar rats at a rate comparable to standard drug diclofenac (82). The anti-inflammatory effect of capsaicin was initially linked to capsaicin receptors known as transient receptor potential vanilloid sub-type1 (TRPV1). Vanilloid receptors have been implicated in tissue injury and inflammation; however, repeated application of capsaicin results in anti-inflammatory responses via these receptors. Recent studies have suggested that the anti-inflammatory action of capsaicin is independent of the TRPV1 receptor (83–85).
Capsaicin mediates anti-inflammation by inhibiting lipopolysaccharide (LPS)-induced IL-1β, IL-6 and TNF-α production by increasing Liver X receptor α (LXRα) expression through peroxisome proliferator-activated receptor-gamma (PPARγ) pathway (85). The authors also observed that the activation of LXRα blocks NF-κB-mediated inflammatory gene expression and the inhibitory action of capsaicin on NF-κB expression was blocked by LXRα inactivation with siRNA. Likewise, capsaicin inhibits toll-like receptor-mediated salivary epithelial cells’ release of pro-inflammatory cytokines through the NF-kB signalling pathway (84). Kim et al. (83) reported the inhibition of NF-kB by capsaicin via a mechanism involving the degradation of ikB-α. The compound elicits COX-2 enzyme activity inhibition and downregulation of iNOS protein to ameliorate inflammation in LPS-stimulated murine peritoneal macrophages. Chen et al. (86) investigated the signal transduction mechanism implicated in the anti-inflammation action of capsaicin in RAW264.7 macrophages. Capsaicin inhibited LPS- and IFN-γ-mediated NO production, iNOS protein and mRNA expression, COX-2 expression and PGE2 production. In addition, capsaicin inhibits NF-κB, AP-1 activation and STAT1 activation, as well as other upstream protein kinases, including ERK, JNK and IKK. The inhibition of the upstream kinase is implicated in the apoptotic action of capsaicin, which is discussed in the subsequent session. The overall anti-inflammatory of capsaicin is summarized in Figure 5.
Cell progresses through the G0/G1, S and G2/M phases of the cell cycle during cell proliferation. This series of events is highly regulated by cyclin, cyclin-dependent kinase, and checkpoint kinases, including polo-like kinase, aurora kinase and CDK inhibitors which ensures that damaged/mutated cells do not proceed through in cell cycle (87). However, in cancer cells, deregulation in cell cycle regulations allows cell proliferation to occur. Over the years, dietary phytochemicals such as capsaicin have shown attractive cell cycle regulation activity, thereby halting cellular division of cancer cells (87). Upon sensitization by proliferative stimulus, cell progresses from the resting G0 phase to the growth phase (G1). A recent study observed that capsaicin mediates cell cycle arrest at the G1 phase in ORL-48 cells (88). Similarly, Qian et al. (89) reported G0/G1 cell cycle arrest in bladder cancer cells following capsaicin treatment. However, studies have also reported the inhibition of the cell cycle at the G2/M phase in human KB cancer cells and MCF7 breast cancer cells. The cellular arrest is usually achieved by modulation of cell cycle protein kinases. For instance, downregulation of CDK8 expression was involved in the G2/M phase arrest of breast cancer cells by capsaicin (90). In another study, the inhibition of CDK2, CDK4 and CDK6 were responsible for G0/G1 arrest (91). Likewise, the anti-tumor effect of capsaicin on human pharyngeal squamous carcinoma cells (FaDu) is associated with mitochondrial pathways, possibly by decreasing the expression of the regulators of cyclin B1 and D1, as well as cyclin-dependent protein kinases CDK-1, CDK-2 and CDK-4 mediating cell cycle arrest at G1/S phase (92).
Beyond the anti-CDK activity of capsaicin, capsaicin modulates upstream molecular events such as the p53 dependent pathways. Islam et al. (93) recently reported that tumour-associated NADH oxidase (tNOX) is a major target of capsaicin responsible for its effect on the cell cycle. The authors noted that modulating tNOX reduces NAD+ generation and inhibits SIRT1, causing c-myc and p53 activation, ultimately leading to the inhibition of cyclin/CDK complex at G1 checkpoint triggering cell cycle arrest. In bladder cancer, capsaicin treatment down-regulates tNOX and SIRT1 expression prolonging cell cycle progression among other effects (94). Capsaicin elicits anticancer effect via a p53 dependent pathway in human colon cancer cells (95). By suppressing p53/MDM2 interaction, capsaicin inhibited p53 degradation, allowing p53 to induce cell cycle arrest at the G0/G1 phase and apoptosis (95). In addition, via a mechanism that involves the vanilloid receptor TRPV1, capsaicin modulates the expression of p53, p21 and CDK2, initiating G0/G1 phase arrest in bladder cancer RT4 cells (96). Overall, by modulating critical signal transducers in the cell cycle, capsaicin can halt cancer proliferation in different cancer types to prevent the progression of cancer.
Apart from being a chemopreventive agent, capsaicin has shown cytotoxic effects. It has been reported to cause the induction of cell death in different cancer cells in in vitro and in vivo models.
Apoptosis is the primary mechanism via which capsaicin can induce cell death in cancer cells, including prostate cancer, pancreatic cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, and skin cancer. Apoptosis is a programmed form of cell death characterized by morphological and biochemical events including membrane blebbing, cell shrinkage, nuclear and DNA fragmentation, chromatin condensation followed by engulfment of the dead cells by neighbouring cells (97). A different molecular mechanism capable of inducing apoptosis has been described following the treatment of cancer cells with capsaicin.
One of the major pro-apoptotic mechanisms of capsaicin is via the vanilloid receptors, primarily TRPV1, a non-selective calcium channel that has been functionally involved in cell death in a wide variety of cancer cells. In glioma cells, capsaicin treatment increased the expression of TRPV1, causing a concomitant influx of Ca2+ triggering apoptosis via the p38 signalling pathway (98). Similarly, in anaplastic thyroid cancer, the agonistic role of capsaicin led to the inhibition of the cell viability as a result of cell death via the intrinsic pathway of apoptosis (99). The mechanism also involved triggering Ca2+ influx into the cell cytoplasm, causing an imbalance in intracellular calcium homeostasis and a severe condition of mitochondria calcium overload (99). The disruption of the mitochondria calcium balance resulted in increased production of mitochondria reactive oxygen species, depolarization of mitochondria membrane potential, and opening of mitochondria membrane permeability pore (99). The latter effects result in the release of cytochrome C, triggering apoptosome assembly and the activation of caspase, leading to apoptotic cell death. Equally worth emphasizing is that the study also showed that in the presence of TRPV1 antagonist and calcium chelator, apoptosis underscores the role of the TRPV1 receptor pathway in capsaicin-induced apoptosis in cancer cells (99).
Amantini et al. (100) studied the role of TRPV1 in capsaicin-induced apoptosis in human urothelial cells. The authors noted that TRPV1 dependent apoptosis involved the activation of pro-apoptotic protein -ataxia-telangiectasia mutated (ATM), which is involved in Ser15, Ser20, and Ser392 phosphorylation in the DNA damage response pathway, and the activation of Fas/CD95 protein which mediates intrinsic and extrinsic apoptosis pathway. Likewise, TRPV1 activation following capsaicin treatment results in apoptosis induction in colorectal cancer via the calcineurin-NFAT2-p53 signaling pathway (101). Aside from TRPV1, another member of the TRPV family which has been implicated in the apoptotic action of capsaicin is TRPV6. Like TRPV1, TRPV6 is a calcium selective ion channel that regulates calcium homeostasis. In human small cell lung cancer, capsaicin displays potent antineoplastic activity by increasing TRPV6 expression, causing increased levels of intracellular calcium ions activating the calpain pathway to induce apoptosis (102). According to Chow et al. (103) TRPV6 mediated capsaicin-induced apoptosis activation in gastric cancer cells. It was observed that TRPV6 overexpression increased mitochondria permeability in the cells through the activation of Bax and p53 through C-jun N-terminal kinase (JNK) activation (Figure 6).
Perhaps, what makes capsaicin a particularly interesting anticancer agent is its ability to act on multiple anticancer targets? This feature is once again exemplified in its apoptotic mechanism, which has been found not to be limited to vanilloid receptor pathways (104). The studies by Bao et al. (105) revealed that by treating human osteosarcoma MG63 cells with capsaicin, apoptosis by induced via the TRPV1-dependent and independent pathways. Through the TRPV1 independent pathway, capsaicin-induced apoptosis by activating adenosine-5-monophosphate-activated protein kinase (AMPK), p53 and JNK. Findings by Kida et al. (106) showed that in the presence of TRPV1 antagonist, capsaicin stimulated intracellular calcium influx. Likewise, the binding of capsaicin to mitochondrial complex I and II in the electron transport chain disrupted the mitochondrial membrane potential and increasing mitochondrial membrane permeability (107). Zhang et al. (108) equally showed that capsaicin increases ROS levels and results in increased expression of pro-apoptotic Bcl-2 (Bax), downregulation of anti-apoptotic Bcl-2 and CytC release, causing cell death in pancreatic cancer cells in vitro and in vivo. Thus, via a mechanism independent of TRPV1, capsaicin can activate the extrinsic and intrinsic apoptosis pathways.
Autophagy is a highly regulated process through which cytoplasmic components are delivered to the lysosome for degradation and later recycled to meet the metabolic needs of starving cells (68, 109). While it was initially thought of as a pro-survival mechanism, autophagy has been found to play a dichotomous role as a cell survival mechanism and cell death mechanism (68, 109). Although the role of capsaicin in autophagic death is yet to be fully understood, it appears to vary with different cancer cell types as it has been shown to inhibit or promote autophagy in different forms of cancer (110–112). By blocking the Pi3/Akt/mTOR signalling pathway, capsaicin increases levels of autophagic markers (LC3-II and Atg5), enhances p62 and Fap-1 degradation and increases caspase-3 activity to induce apoptosis in human nasopharyngeal carcinoma cells (112). Moreover, capsaicin acts through tNOX to induce autophagic apoptosis in oral and melanoma cancer cells (113, 114). Conversely, in U251 Glioma cells, capsaicin was shown to inhibit autophagy, and this inhibition resulted in increased apoptotic cell death (110). As such, in these cancer cells, autophagy appears to be a pro-survival mechanism and its inhibition by capsaicin results in cell death.
However, most studies have suggested that capsaicin is likely to induce autophagy in cancer cells in a manner that assists in cancer cell survival. For instance, in the study by Chu et al. (111), it was shown that capsaicin-induced autophagy, which served a tumour-promoting role in human melanoma cells and inhibition of autophagy using 3-MA enhanced capsaicin-induced cell death. Similarly, Chen et al. (115) increased stat3 dependent autophagy through the generation of ROS in human hepatoma (HepG2 cells) and inhibition of autophagy enhanced capsaicin-induced apoptosis. Further studies have shown that autophagy induction by capsaicin retards cell death by suppressing endoplasmic reticulum stress-mediated apoptosis via a pathway involving JNK, p38 and ERK (116, 117).
Although apoptosis is the main form of cell death described in capsaicin-treated cancer cells, other forms of cell death such as necrosis, paraptosis, and necroptosis have also been reported using in vitro and in vivo models (118, 119). Several factors, including cell type, dose and time of capsaicin exposure, can influence the type of cell death mediated by capsaicin (118). Capsaicin induces necrotic cell death in a time-dependent manner in 5637 and T24 BC cells and that autophagic inhibitor enhances the cytotoxic effects (120). Following the investigation of the effect of capsaicin-induced TRPV1 expression on cell proliferation in breast cancer, Wu et al. (121) observed that TRPV1 does not enhance cell proliferation and capsaicin was able to induce necrotic cell death in the MCF-7 cell, which was associated with increased expression of c-fos and RIP3. Ramírez-Barrantes et al. (122) equally established that TRPV1 expression mediates necrosis in HeLa cells. The authors observed that at high concentration (> 10 µM), capsaicin induces a slow but persistent increase in intracellular Ca2+, which leads to plasma membrane depolarization, mitochondrial dysfunction, and ultimately cell death by necrosis and apoptosis.
Another form of cell death has been described by Jambrina et al. (119), using capsaicin. The authors reported that activation of TRPV1 by capsaicin causes Ca2+ influx, which triggers a distinct program of mitochondrial dysfunction leading to paraptotic cell death, which does not fulfil the criteria for either apoptosis or necrosis. Huang et al. (123) also reported necroptosis (programmed necrosis) in oral squamous cell carcinoma cells treated with capsaicin. It is noteworthy that the role of paraptosis and necroptosis in the anticancer effects of capsaicin is yet to be fully elucidated. Further studies are thus required to carefully study this pathway of cell death in capsaicin-treated anticancer cells.
Tumor cells, in certain cases, are capable of migrating through the lymphatic or blood systems to colonize distant sites through a process known as metastasis. This is a complex process implicated in 90% of cases of cancer mortalities, and it involves several alterations causing stimulation of angiogenesis, local invasion attachment, basement membrane disruption, matrix proteolysis, and stimulation of growth factors (68, 124). Capsaicin has been shown in the past to mitigate cancer metastasis due to the ability to modulate critical pathways involved in molecular alterations of cancer. For instance, Min et al. (125) described the inhibition of angiogenesis by capsaicin under in vitro and in vivo systems. Angiogenesis is the formation of new blood vessels to deliver nutrients and oxygen necessary for secondary tumor growth. By inhibiting angiogenesis, capsaicin can stall the growth of secondary tumours. In non-small lung cancer cells, capsaicin was able to restrain angiogenesis by dampening vascular endothelial growth factor (VEGF) expression via p53-SMAR1 auto-regulatory loop activation (126). Capsaicin is also able to inhibit tumor metastasis by inhibiting the matrix proteolysis pathway. Specifically, capsaicin has been shown to target matrix metalloproteinase 9 (MMP9), a protein responsible for extracellular matrix degradation and cytokine activation during tissue remodelling in metastatic cancer. The inhibition of MMP9 by capsaicin occurs via the suppression of AMPK-NF-κB, EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling pathway (127, 128). In addition, Shin et al. (129) outlined phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway inhibition as the primary mechanism of cell migration in B16-F10 melanoma. In human papillary thyroid carcinoma BCPAP cells, capsaicin inhibits matrix protease MMP9 and MMP2 by activating the TRPV1 channel (130). Based on recent evidence (131), capsaicin might inhibit migration and invasion and metastasis of oesophageal squamous cell carcinoma (ESCC) via overexpression of claudin-3 (Cldn3) and inhibiting epithelial-mesenchymal transition (EMT). The anti-metastatic effect of capsaicin has been further validated using in vivo mouse prostate cancer model where it was demonstrated that capsaicin significantly reduced the metastatic burden (132).
Within the last two decades, there have been several clinical reports on the use of capsaicin. However, most of the studies have mainly examined the analgesic activity of capsaicin. There have been few reports on the use of capsaicin in cancer patients; however, these studies have examined the pain relief function of capsaicin in addition to other treatment regimens (133–135). For instance, Adlea (ALRGX-4975) - an injectable preparation of capsaicin in phase II clinical trials in Morton’s neuroma patients, was effective in the treatment of chronic neuropathic pain (134). Likewise, Privitera and Anand (133) revealed that capsaicin 8% patch could promote the regeneration and restoration of skin nerve fibres in chemotherapy-induced peripheral neuropathy in addition to pain relief. These studies have suggested that capsaicin might serve as a suitable adjuvant to ameliorate pain and its associated complications in cancer treatment. However, it remains unclear whether capsaicin serves any antiproliferative function in cancer patients. Further clinical studies focused on its antiproliferative potential are therefore required.
The last few decades have seen a significant increase in the number of research conducted on the anticancer effects of capsaicin, yet none of these research works has been met by clinical approval. This limitation stems from certain challenges with capsaicin as a drug. One of the limitations of capsaicin is its pro-carcinogenic effects. Although the number of anticancer studies of capsaicin far outweighs the carcinogenic activity, nonetheless, the risk associated with capsaicin carcinogenic effect is a major concern to researchers (136). Another major drawback of capsaicin is the lead-likeness property. Capsaicin has been shown to have high hydrophobicity, low binding affinity, and short half-life, which can affect the in vivo anticancer efficacy (31). More so, capsaicin has shown several unpleasant side effects, including stomach cramps, skin and gastric irritation, and burning sensation (137). Hence, we further discuss the recent studies carried out to improve the anticancer efficacy of capsaicin.
The majority of capsaicin used in research have been purified from Capsicum plants, with varying levels of purity which has led to a disparity in some results obtained from biological assays (138). To circumvent these challenges, capsaicin has been synthesized artificially with a high level of purity and high yield. Furthermore, to bypass some side effects and limitations with capsaicin, different capsaicin analogs have been synthesized some of which have shown significant anticancer prospect. A typical capsaicin structure consists of an aromatic ring (region A), an amide group (region B), and a hydrophobic group (region C) (137). Modifications of capsaicin pharmacophore have focused mainly on the B and C region of the capsaicin structure to yield capsaicinoids such as capsiate, dehydrocapsaite, nordihydrocapsiate, which have only shown anticancer properties without any reported carcinogenic effects (137). In a study by Lewinska et al. (139), capsaicin epoxide was synthesized and found to be non-toxic to human dermal fibroblast cell lines and showed higher toxicity to cancer cell lines compared to capsaicin by inducing oxidative damage. Likewise, by modifying regions A and B of capsaicin, Pereira et al. (140) synthesized a capsaicin-like analogue which induced apoptotic cell death in cancer cells with a better pharmacokinetic profile than capsaicin and had no irritant effects on mice. These findings were corroborated by de-Sá-Júnior et al. (141), who synthesized RPF101, by modifying similar aromatic and amide substituent groups. The compound had a better pharmacokinetic profile than capsaicin and was reactive toward the cancer target. In addition, capsazepine − a TRPV1 antagonist, has also been synthesized and found to be a highly effective pleitropic antitumoral/anti-inflammatory agent in cancer cells and in vivo models (142). So far, the several capsaicin analogs have shown significant promise but require further in vivo and clinical validations.
In an effort to enhance the bioavailability, improve the pharmacokinetics and half-life and reduce the side effects, different delivery vehicles, including inorganic carriers (metal nanoparticles and carbon sphere), polymeric carriers (micelle, dendrimer and polymersome), and lipid-based nanoparticles (liposomes, microencapsulation and solid-lipid nanoparticle) have been developed to perform site-directed delivery of capsaicin (143). In addition, excipient-free self-assembled capsaicin delivery systems have been designed with improved pharmacokinetic properties (144). Studies on the delivery of capsaicin for improved anticancer functions are summarized in Table 1.
Delivery systems such as nanoparticles offer the advantage of increasing the retention time in the blood system, thereby allowing the drug to achieve maximum efficacy before being cleared from the body. Likewise, liposomes and micro-emulsion-based drugs have been known to significantly improve oral bioavailability and reduce the irritation of drugs (154). In addition, these delivery systems can be surfaced-modified to perform site-directed/cell-specific drug delivery, thereby ensuring increased cell death of cancer cells while sparing non-selective normal cells (155). Furthermore, owing to its antioxidant potential, capsaicin has been applied asa bioreduction and capping agent to synthesize biocompatible silver nanoparticles and can be used in cancer theranostics (156).
The current generation of cancer therapeutics has good initial efficacy but often develops resistance within months of treatment. One way of combating this problem is through drug-drug combination and combination of chemotherapeutic treatment with other anticancer therapies such as radiotherapy and photothermal therapy. In the same vein, capsaicin has been combined with other anticancer therapies for more pronounced anticancer effects (Table 2). In most cases, the combination of capsaicin with other chemotherapeutic drugs has shown a significant synergistic effect. Recent studies have shown that capsaicin may also serve additional benefits as an adjunct to current chemotherapeutic drugs. The unique TRPV1 dependent cell death mechanism of capsaicin together with other cell death pathways by chemotherapeutic drugs ensures complete clearance of the cancer cells and makes it less likely for cancer cells to develop resistance.
Evidence from this review highlighted the research trend and pattern (e.g., top authors, journals, and publications). It revealed an in-depth insight into the potential of capsaicin for managing human cancer. The last two decades have witnessed an increasing rate (an average of ca. 18% growth yearly) in the number of publications (dominated by research articles at 93%) following the renewed interest in capsaicin research. The research outputs have been majorly (ca. 42% of 3753 publications) produced by the United States, China, and Japan, which also had a visibly dominant collaboration node and network with most of the other countries identified in this review. Despite the evident productive collaboration, the inadequate representation of countries from the developing world remains a concern that needs to be addressed for significant success in exploring the potential of capsaicin for mitigating human cancer. The importance of concerted effort toward developing research collaboration between developed and developing countries cannot be over-emphasised. Based on the assessed eligible literature, the four keyword clusters generated and designated as thematic domains for capsaicin research included anti-cancer/pharmacokinetics, cytotoxicity, and in vivo neurological and pain research studies. The top-20 publications were distributed across multiple science-based subjects such as neurosciences, pharmacology, biochemistry, physiology, chemistry, cell biology, food science and technology, thereby suggesting the multidisciplinary approach currently being explored for capsaicin as potential therapeutics for several health conditions. In relation to the top-20 cited publications, the anti-cancer/pharmacokinetics/pharmacodynamics of capsaicin was the most active thematic domain in the last two decades.
The potential of capsaicin for mitigating cancer has been mainly explored for its chemopreventive effects and mechanisms involved in cell death as well as intervention in cancer metastasis. The chemopreventive effect of capsaicin is related to its ability to exert diverse biological effects, including anti-mutagenic, antioxidant and anti-inflammatory activities as well as cell cycle regulation. Furthermore, capsaicin demonstrated cytotoxic effects, often facilitated by the induction of cell death in different cancer cells under in vitro and in vivo models. Overall, capsaicin has shown effectiveness against various human malignancies in recent years. Although there is an increasing focus on assessing the clinical effects of capsaicin, especially the analgesic activity, the anticancer efficacy is currently limited. This has been attributed to the pro-carcinogenic effect, high hydrophobicity, low binding affinity and short half-life of capsaicin. Hence, more research efforts geared at mitigating these limitations remain pertinent. Some of the currently applied approaches entail synthesising natural and synthetic analogs, precise targeted delivery, drug synergism, and combination therapy for capsaicin. The potential of combination therapy for improved anticancer properties, especially for lung and prostate cancer, has demonstrated some promising results, which indicates the therapeutic value of capsaicin. Studies are required to identify capsaicin analogs with long-acting and greater anticancer effects. It is envisaged that promising results from these ongoing approaches (that address the existing limitations) will surely feed into future clinical studies on the anti-proliferative potential of capsaicin.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
TA acknowledges post-doctoral fellowship from the North-West University, South Africa. Support in form of the APC from the Faculty of Natural and Agricultural Sciences, North-West University is sincerely appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the University of KwaZulu-Natal, Nelson Mandela University and University of Cape Town for institutional support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.908487/full#supplementary-material
Supplementary Table 1 | Main information on global capsaicin-related research from 2001 to 2021.
Supplementary Table 2 | Top 20 author keywords (DE) and keywords-plus (ID) associated with capsaicin articles from 2001 to 2021.
Supplementary Table 3 | Top 20 journal sources (of the 3753 articles) for capsaicin research from 2001 to 2021.
Supplementary Table 4 | Leading authors in capsaicin research from 2001 to 2021 and their citation analysis.
Supplementary Table 5 | Top 20 most cited research articles on capsaicin from 2001 to 2021 based on total citations (TC) and total citation per year (TC/Year).
Supplementary Table 6 | Twenty top leading and most cited countries of capsaicin publications from 2001 to 202.
Supplementary Figure 1 | Chemical structure of capsaicin.
Supplementary Figure 2 | Capsaicin biosynthetic pathways (30, 174). PAL-phenylalanine ammonia lyase, CIAH-cinnamic acid 4-hydroxylase, COAH-coumaric acid 3- hydroxylase, CAMT-caffeic acid O-methyltransferase, PAT-putative aminotransferase, BCAT- branched chain amino acid transferase, IVD- isovalerate deshidrogenase, FAS-fatty acid synthase complex, KAS-β-ketoacyl-[acyl-carrier-protein] (ACP) synthase, ACL-acyl carrier protein, TATA- acyl-ACP thioesterase, ACS-acyl-CoA synthase, CS-capsaicin synthase.
Supplementary Figure 3 | Flow chart of the bibliometric study of capsaicin showing the selection and inclusion criteria from Web of Science (WoS) and Scopus databases.
Supplementary Figure 4 | Twenty most productive authors in capsaicin research (2001–2021).
Supplementary Figure 5 | Network visualization map showing country collaborations on capsaicin research. The node represents countries. Node diameter signifies the collaboration strength of a country with other countries. Lines depict collaboration pathways between countries. Korea (North Korea and South Korea).
1. Olatunji TL, Afolayan AJ. Comparison of Nutritional, Antioxidant Vitamins and Capsaicin Contents in Capsicum Annuum and C. Frutescens. Int J Veg Sci (2020) 26:190–207. doi: 10.1080/19315260.2019.1629519
2. Wang F, Xue Y, Fu L, Wang Y, He M, Zhao L, et al. Extraction, Purification, Bioactivity and Pharmacological Effects of Capsaicin: A Review. Crit Rev Food Sci Nutr (2021) 1–29. doi: 10.1080/10408398.2021.1884840
3. Hayman M, Kam PCA. Capsaicin: A Review of its Pharmacology and Clinical Applications. Curr Anaesth Crit Care (2008) 19:338–43. doi: 10.1016/j.cacc.2008.07.003
4. Nelson EK, Dawson DE. The Constitution of Capsaicin, the Pungent Principle of. Capsicum. III. J Am Chem Soc (1923) 45:2179–81. doi: 10.1021/ja01662a023
5. Späth E, Darling SF. Synthese Des Capsaicins. Berichte der Dtsch Chem Gesellschaft(A B Ser (1930) 63:737–43. doi: 10.1002/cber.19300630331
6. De Lourdes Reyes-Escogido M, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and Pharmacological Aspects of Capsaicin. Molecules (2011) 16:1253–70. doi: 10.3390/molecules16021253
7. Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S. A Comprehensive Review of the Carcinogenic and Anticarcinogenic Potential of Capsaicin. Toxicol Pathol (2012) 40:847–73. doi: 10.1177/0192623312444471
8. Cheema SK, Pant MR. Estimation of Capsaicin in Seven Cultivated Varieties of Capsicum Annuum L. (2011) (Accessed March 9, 2022).
9. Supalkova V, Stavelikova H, Krizkova S, Adam V, Horna A, Havel L, et al. Study of Capsaicin Content in Various Parts of Pepper Fruit by Liquid Chromatography With Electrochemical Detection. (2007) (Accessed March 9, 2022).
10. Sung Y, Chang YY, Ting NL. Capsaicin Biosynthesis in Water-Stressed Hot Pepper Fruits. (2005) (Accessed March 8, 2022).
11. Arora R, Gill N, Chauhan G, Rana A. An Overview About Versatile Molecule Capsaicin. Int J Pharm Sci Drug Res (2011) 3:280–6.
12. Castillo E, López-González I, De Regil-Hernández R, Reyes-Duarte D, Sánchez-Herrera D, López-Munguía A, et al. Enzymatic Synthesis of Capsaicin Analogs and Their Effect on the T-Type Ca2+ Channels. Biochem Biophys Res Commun (2007) 356:424–30. doi: 10.1016/j.bbrc.2007.02.144
13. Choi HY, Yoon SH. Bioisoster of Capsaicin: Synthesis of 1-Hydroxy-2-Pyridone Analogue. (1999) (Accessed March 8, 2022).
14. Ochoa-Alejo N, Ramirez-Malagon R. In Vitro Chili Pepper Biotechnology. Vitr Cell Dev Biol - Plant (2001) 37:701–29. doi: 10.1007/s11627-001-0121-z
15. Pandhair V, Gosal SS. Capsaicin Production in Cell Suspension Cultures Derived From Placenta of Capsicum Annuum L. (2009) (Accessed March 8, 2022).
16. Akhtar F, Sharif HM, Mallick MA, Zahoor F, Abdulmalik A, Baig W, et al. Capsaicin: Its Biological Activities and in Silico Target Fishing. (2017) (Accessed March 8, 2022).
17. Ochoa-Alejo N, Gómez-Peralta JE. Activity of Enzymes Involved in Capsaicin Biosynthesis in Callus Tissue and Fruits of Chili Pepper (Capsicum Annuum L.). J Plant Physiol (1993) 141:147–52. doi: 10.1016/S0176-1617(11)80751-0
18. Scheau C, Badarau IA, Caruntu C, Mihai GL, Didilescu AC, Constantin C, et al. Capsaicin: Effects on the Pathogenesis of Hepatocellular Carcinoma. Molecules (2019) 24:2350. doi: 10.3390/molecules24132350
19. Xiang Q, Guo W, Tang X, Cui S, Zhang F, Liu X, et al. Capsaicin—the Spicy Ingredient of Chili Peppers: A Review of the Gastrointestinal Effects and Mechanisms. Trends Food Sci Technol (2021) 116:755–65. doi: 10.1016/j.tifs.2021.08.034
20. O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the Mystery of Capsaicin: A Tool to Understand and Treat Pain. Pharmacol Rev (2012) 64:939–71. doi: 10.1124/pr.112.006163
21. Rollyson WD, Stover CA, Brown KC, Perry HE, Stevenson CD, McNees CA, et al. Bioavailability of Capsaicin and Its Implications for Drug Delivery. J Control Release (2014) 196:96–105. doi: 10.1016/j.jconrel.2014.09.027
22. Fernandes ES, Cerqueira ARA, Soares AG, Costa SKP. Capsaicin and Its Role in Chronic Diseases. Adv Exp Med Biol (2016) Springer:91–125. doi: 10.1007/978-3-319-41342-6_5
23. Goswami A. Capsaicin. J Pain Palliat Care Pharmacother (2012) 26:373–5. doi: 10.3109/15360288.2012.734901
24. Singh U, Bernstein JA. Intranasal Capsaicin in Management of Nonallergic (Vasomotor) Rhinitis. Prog Drug Res (2014) 68:147–70. doi: 10.1007/978-3-0348-0828-6_6
25. Mori A, Lehmann S, O’Kelly J, Kumagai T, Desmond JC, Pervan M, et al. Capsaicin, a Component of Red Peppers, Inhibits the Growth of Androgen-Independent, P53 Mutant Prostate Cancer Cells. Cancer Res (2006) 66:3222–9. doi: 10.1158/0008-5472.CAN-05-0087
26. Basith S, Cui M, Hong S, Choi S. Harnessing the Therapeutic Potential of Capsaicin and its Analogues in Pain and Other Diseases. Molecules (2016) 21:966. doi: 10.3390/molecules21080966
27. Doherty MJ. Capsaicin Responsiveness and Cough in Asthma and Chronic Obstructive Pulmonary Disease. Thorax (2000) 55:643–9. doi: 10.1136/thorax.55.8.643
28. Ohnuki K, Niwa S, Maeda S, Inoue N, Yazawa S, Fushiki T. CH-19 Sweet, a non-Pungent Cultivar of Red Pepper, Increased Body Temperature and Oxygen Consumption in Humans. Biosci Biotechnol Biochem (2001) 65:2033–6. doi: 10.1271/bbb.65.2033
29. Oyagbemi A, Saba A, Azeez O. Capsaicin: A Novel Chemopreventive Molecule and its Underlying Molecular Mechanisms of Action. Indian J Cancer (2010) 47:53. doi: 10.4103/0019-509X.58860
30. Chapa-Oliver AM, Mejía-Teniente L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules (2016) 21:931. doi: 10.3390/MOLECULES21080931
31. Zhang S, Wang D, Huang J, Hu Y, Xu Y. Application of Capsaicin as a Potential New Therapeutic Drug in Human Cancers. J Clin Pharm Ther (2020) 45:16–28. doi: 10.1111/jcpt.13039
32. Surh Y-J, Lee SS. Capsaicin in Hot Chili Pepper: Carcinogen, Co-Carcinogen or Anticarcinogen? Food Chem Toxicol (1996) 34:313–6. doi: 10.1016/0278-6915(95)00108-5
33. Castro-Muñoz R, Gontarek-Castro E, Jafari SM. Up-To-Date Strategies and Future Trends Towards the Extraction and Purification of Capsaicin: A Comprehensive Review. Trends Food Sci Technol (2022) 123:161–71. doi: 10.1016/j.tifs.2022.03.014
34. Wu Q, Bai P, Guo H, Guo MSS, Xia Y, Xia Y, et al. Capsaicin, a Phytochemical From Chili Pepper, Alleviates the Ultraviolet Irradiation-Induced Decline of Collagen in Dermal Fibroblast via Blocking the Generation of Geactive Gxygen Species. Front Pharmacol (2022) 13:872912. doi: 10.3389/fphar.2022.872912
35. Toukan N, Kulnik ST, Lewko A, ElShaer A. Therapeutic Applications of Capsaicin in Humans to Target Conditions of the Respiratory System: A Scoping Review. Respir Med (2022) 194:106772. doi: 10.1016/j.rmed.2022.106772
36. Adetunji TL, Olisah C, Adegbaju OD, Olawale F, Adetunji AE, Siebert F, et al. The Genus Aloe: A Bibliometric Analysis of Global Research Outputs (2001–2020) and Summary of Recent Research Reports on its Biological Activities. South Afr J Bot (2022)147:953–975. doi: 10.1016/j.sajb.2022.01.030
37. Olatunji TL, Adetunji AE, Olisah C, Idris OA, Saliu OD, Siebert F. Research Progression of the Genus Merremia: A Comprehensive Review on the Nutritional Value, Ethnomedicinal Uses, Phyto-Chemistry, Pharmacology, and Toxicity. Plants (2021) 10:2070. doi: 10.3390/plants10102070
38. Balstad MT, Berg T. A Long-Term Bibliometric Analysis of Journals Influencing Management Accounting and Control Research. J Manag Control (2020) 30:357–80. doi: 10.1007/s00187-019-00287-8
39. Tarragona J, de Gracia A, Cabeza LF. Bibliometric Analysis of Smart Control Applications in Thermal Energy Storage Systems. A Model predictive control approach. J Energy Storage (2020) 32:101704. doi: 10.1016/j.est.2020.101704
40. Joshi A. Comparison Between Scopus & ISI Web of Science. J Glob Values ISSN (2016) VII:976–9447.
41. Patowary P, Pathak MP, Zaman K, Raju PS, Chattopadhyay P. Research Progress of Capsaicin Responses to Various Pharmacological Challenges. BioMed Pharmacother (2017) 96:1501–12. doi: 10.1016/J.BIOPHA.2017.11.124
42. Goerlandt F, Li J. Forty Years of Risk Analysis: A Scientometric Overview. Risk Anal (2021) 00:2021. doi: 10.1111/RISA.13853
43. Vadivelu N, Mitra S, Narayan D. Recent Advances in Postoperative Pain Management. Yale J Biol Med (2010) 83:11–25.
44. O’Neill TP. Mechanism of Capsaicin Action: Recent Learnings. Respir Med (1991) 85:35–41. doi: 10.1016/S0954-6111(06)80252-0
45. Hall OM, Broussard A, Range T, Carroll Turpin MA, Ellis S, Lim VM, et al. Novel Agents in Neuropathic Pain, the Role of Capsaicin: Pharmacology, Efficacy, Side Effects, Different Preparations. Curr Pain Headache Rep (2020) 24:53. doi: 10.1007/s11916-020-00886-4
46. Panpetch W, Visitchanakun P, Saisorn W, Sawatpanich A, Chatthanathon P, Somboonna N, et al. Lactobacillus Rhamnosus Attenuates Thai Chili Extracts Induced Gut Inflammation and Dysbiosis Despite Capsaicin Bactericidal Effect Against the Probiotics, a Possible Toxicity of High Dose Capsaicin. PloS One (2021) 16:e0261189. doi: 10.1371/journal.pone.0261189
47. Lin CH, Lu WC, Wang CW, Chan YC, Chen MK. Capsaicin Induces Cell Cycle Arrest and Apoptosis in Human KB Cancer Cells. BMC Complement Altern Med (2013) 13:1–9. doi: 10.1186/1472-6882-13-46
48. Chung M-K, Campbell J. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals (2016) 9:66. doi: 10.3390/ph9040066
49. Yong YL, Tan LTH, Ming LC, Chan KG, Lee LH, Goh BH, et al. The Effectiveness and Safety of Topical Capsaicin in Postherpetic Neuralgia: A Systematic Review and Meta-Analysis. Front Pharmacol (2017) 7:538. doi: 10.3389/fphar.2016.00538
50. Aksnes DW, Langfeldt L, Wouters P. Citations, Citation Indicators, and Research Quality: An Overview of Basic Concepts and Theories. SAGE Open (2019) 9:215824401982957. doi: 10.1177/2158244019829575
51. Li W, Zhao Y. Bibliometric Analysis of Global Environmental Assessment Research in a 20-Year Period. Environ Impact Assess Rev (2015) 50:158–66. doi: 10.1016/j.eiar.2014.09.012
52. van Raan AFJ. Comparison of the Hirsch-Index With Standard Bibliometric Indicators and With Peer Judgment for 147 Chemistry Research Groups. Scientometrics (2006) 67:491–502. doi: 10.1556/Scient.67.2006.3.10
53. Tahim A, Patel K, Bridle C, Holmes S. The 100 Most Cited Articles in Facial Trauma: A Bibliometric Analysis. J Oral Maxillofac Surg (2016) 74:e1–2240.e14:2240. doi: 10.1016/j.joms.2016.06.175
54. Amadesi S. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J Neurosci (2004) 24:4300–12. doi: 10.1523/JNEUROSCI.5679-03.2004
55. Ghilardi JR. Selective Blockade of the Capsaicin Receptor Trpv1 Attenuates Bone Cancer Pain. J Neurosci (2005) 25:3126–31. doi: 10.1523/JNEUROSCI.3815-04.2005
56. Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and Nerve Growth Factor Release the Capsaicin Receptor From PtdIns(4,5)P2-Mediated Inhibition. Nat 2001 4116840 (2001) 411:957–62. doi: 10.1038/35082088
57. Petersen OH. Inequality of Research Funding Between Different Countries and Regions is a Serious Problem for Global Science. Function (2021) 2:60. doi: 10.1093/function/zqab060
58. Fadelu T, Rebbeck TR. The Rising Burden of Cancer in Low– and Middle–Human Development Index Countries. Cancer (2021) 127:2864–6. doi: 10.1002/cncr.33586
59. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
60. The Cancer Atlas. The Burden of Cancer, in: The Cancer Atlas (2019). Available at: https://canceratlas.cancer.org/the-burden/the-burden-of-cancer/ (Accessed March 3, 2022).
61. Popescu GDA, Scheau C, Badarau IA, Dumitrache M-D, Caruntu A, Scheau A-E, et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules (2020) 26:94. doi: 10.3390/molecules26010094
62. Agbarya A, Ruimi N, Epelbaum R, Ben-Arye E, Mahajna J. Natural Products as Potential Cancer Therapy Enhancers: A Preclinical Update. SAGE Open Med (2014) 2:205031211454692. doi: 10.1177/2050312114546924
63. Gosslau A, Li S, Ho C-T, Chen KY, Rawson NE. The Importance of Natural Product Characterization in Studies of Their Anti-Inflammatory Activity. Mol Nutr Food Res (2011) 55:74–82. doi: 10.1002/mnfr.201000455
64. Key TJ, Allen NE, Spencer EA, Travis RC. The Effect of Diet on Risk of Cancer. Lancet (2002) 360:861–8.
65. Clark R, Lee SH. Anticancer Properties of Capsaicin Against Human Cancer(2016) (Accessed March 4, 2022).
66. Calixto JB. The Role of Natural Products in Modern Drug Discovery. Acad Bras Cienc (2019) 91:e20190105. doi: 10.1590/0001-3765201920190105
67. Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural Products in Drug Discovery: Advances and Opportunities. Nat Rev Drug Discov (2021) 20:200–16. doi: 10.1038/s41573-020-00114-z
68. Elekofehinti OO, Iwaloye O, Olawale F, Ariyo EO. Saponins in Cancer Treatment: Current Progress and Future Prospects. Pathophysiology (2021) 28:250–72. doi: 10.3390/pathophysiology28020017
69. Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Asokkumar S, Naveenkumar C, et al. Capsaicin Inhibits Benzo (a) Pyrene-Induced Lung Carcinogenesis in an In Vivo Mouse Model. Inflammation Res (2012) 61:1169–75.
70. Ahmad A, Sakr W A, Wahidur Rahman KM. Anticancer Properties of Indole Compounds: Mechanism of Apoptosis Induction and Role in Chemotherapy. Curr Drug Targets (2010) 11:652–66. doi: 10.2174/138945010791170923
71. Han EH, Hwang YP, Kim HG, Choi JH, Park BH, Song GY, et al. CCAAT/ Enhancer-Binding Protein β Activation by Capsaicin Contributes to the Regulation of CYP1A1 Expression, Mediated by the Aryl Hydrocarbon Receptor. Br J Pharmacol (2011) 164:1600–13. doi: 10.1111/j.1476-5381.2011.01232.x
72. Lavorgna M, Orlo E, Nugnes R, Piscitelli C, Russo C, Isidori M. Capsaicin in Hot Chili Peppers: In Vitro Evaluation of its Antiradical, Antiproliferative and Apoptotic Activities. Plant Foods Hum Nutr (2019) 74:164–70. doi: 10.1007/s11130-019-00722-0
73. Sora GTS, Haminiuk CWI, da Silva MV, Zielinski AAF, Gonçalves GA, Bracht A, et al. A Comparative Study of the Capsaicinoid and Phenolic Contents and In Vitro Antioxidant Activities of the Peppers of the Genus Capsicum: An Application of Chemometrics. J Food Sci Technol (2015) 52:8086–94. doi: 10.1007/s13197-015-1935-8
74. Rosa A, Deiana M, Casu V, Paccagnini S, Appendino G, Ballero M, et al. Antioxidant Activity of Capsinoids. J Agric Food Chem (2002) 50:7396–401.
75. Chen K-S, Chen P-N, Hsieh Y-S, Lin C-Y, Lee Y-H, Chu S-C. Capsaicin Protects Endothelial Cells and Macrophage Against Oxidized Low-Density Lipoprotein-Induced Injury by Direct Antioxidant Action. Chem Biol Interact (2015) 228:35–45. doi: 10.1016/j.cbi.2015.01.007
76. Park SG, Yon J, Lin C, Gwon LW, Lee J, Baek I, et al. Capsaicin Attenuates Spermatogenic Cell Death Induced by Scrotal Hyperthermia Through its Antioxidative and Anti-Apoptotic Activities. Andrologia (2017) 49:e12656. doi: 10.1111/and.12656
77. Shimeda Y, Hirotani Y, Akimoto Y, Shindou K, Ijiri Y, Nishihori T, et al. Protective Effects of Capsaicin Against Cisplatin-Induced Nephrotoxicity in Rats. Biol Pharm Bull (2005) 28:1635–8. doi: 10.1159/000365852
78. Tanrıkulu-Küçük S, Başaran-Küçükgergin C, Seyithanoğlu M, Doğru-Abbasoğlu S, Koçak H, Beyhan-Özdaş Ş, et al. Effect of Dietary Curcumin and Capsaicin on Testicular and Hepatic Oxidant–Antioxidant Status in Rats Fed a High-Fat Diet. Appl Physiol Nutr Metab (2019) 44:774–82. doi: 10.1139/apnm-2018-0622
79. Lee J-G, Yon J-M, Lin C, Jung AY, Jung KY, Nam S-Y. Combined Treatment With Capsaicin and Resveratrol Enhances Neuroprotection Against Glutamate-Induced Toxicity in Mouse Cerebral Cortical Neurons. Food Chem Toxicol (2012) 50:3877–85. doi: 10.1016/j.fct.2012.08.040
80. Joung E-J, Li M-H, Lee HG, Somparn N, Jung YS, Na H-K, et al. Capsaicin Induces Heme Oxygenase-1 Expression in HepG2 Cells via Activation of PI3K-Nrf2 Signaling: NAD (P) H: Quinone Oxidoreductase as a Potential Target. Antioxid Redox Signal (2007) 9:2087–98. doi: 10.1089/ars.2007.1827
81. Olawale F, Olofinsan K, Iwaloye O. Biological Activities of Chromolaena Odorata: A Mechanistic Review. South Afr J Bot (2022) 144:44–57. doi: 10.1016/j.sajb.2021.09.001
82. Jolayemi AT, Ojewole JAO. Comparative Anti-Inflammatory Properties of Capsaicin and Ethyl-Aacetate Extract of Capsicum Frutescens Linn [Solanaceae] in Rats. Afr Health Sci (2013) 13:357–61. doi: 10.4314/ahs.v13i2.23
83. Tang J, Luo K, Li Y, Chen Q, Tang D, Wang D, et al. Capsaicin Attenuates LPS-Induced Inflammatory Cytokine Production by Upregulation of Lxrα. Int Immunopharmacol (2015) 28:264–9. doi: 10.1016/j.intimp.2015.06.007
84. Shin Y-H, Namkoong E, Choi S, Bae J-S, Jin M, Hwang S-M, et al. Capsaicin Regulates the NF-κb Pathway in Salivary Gland Inflammation. J Dent Res (2013) 92:547–52. doi: 10.1177/0022034513487376
85. Kim C-S, Kawada T, Kim B-S, Han I-S, Choe S-Y, Kurata T, et al. Capsaicin Exhibits Anti-Inflammatory Property by Inhibiting IkB-A Degradation in LPS-Stimulated Peritoneal Macrophages. Cell Signal (2003) 15:299–306. doi: 10.1016/S0898-6568(02)00086-4
86. Chen C, Lee ST, Wu WT, Fu W, Ho F, Lin WW. Signal Transduction for Inhibition of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Induction by Capsaicin and Related Analogs in Macrophages. Br J Pharmacol (2003) 140:1077–87. doi: 10.1038/sj.bjp.0705533
87. Meeran SM, Katiyar SK. Cell Cycle Control as a Basis for Cancer Chemoprevention Through Dietary Agents. Front Biosci J Virtual Libr (2008) 13:2191. doi: 10.2741/2834
88. Kamaruddin MF, Hossain MZ, Mohamed Alabsi A, Mohd Bakri M. The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48. Medicina (B Aires) (2019) 55:322. doi: 10.3390/medicina55070322
89. Qian K, Wang G, Cao R, Liu T, Qian G, Guan X, et al. Capsaicin Suppresses Cell Proliferation, Induces Cell Cycle Arrest and ROS Production in Bladder Cancer Cells Through FOXO3a-Mediated Pathways. Molecules (2016) 21:1406. doi: 10.3390/molecules21101406
90. Wu D, Jia H, Zhang Z, Li S. Capsaicin Suppresses Breast Cancer Cell Viability by Regulating the CDK8/PI3K/Akt/Wnt/β−Catenin Signaling Pathway. Mol Med Rep (2020) 22:4868–76. doi: 10.3892/mmr.2020.11585
91. Chen D, Yang Z, Wang Y, Zhu G, Wang X. Capsaicin Induces Cycle Arrest by Inhibiting Cyclin-Dependent-Kinase in Bladder Carcinoma Cells. Int J Urol (2012) 19:662–8. doi: 10.1111/j.1442-2042.2012.02981.x
92. Le T-D, Jin D, Rho SR, Kim MS, Yu R, Yoo H. Capsaicin-Induced Apoptosis of FaDu Human Pharyngeal Squamous Carcinoma Cells. Yonsei Med J (2012) 53:834–41. doi: 10.3349/ymj.2012.53.4.834
93. Islam A, Su AJ, Zeng Z-M, Chueh PJ, Lin M-H. Capsaicin Targets tNOX (ENOX2) to Inhibit G1 Cyclin/CDK Complex, as Assessed by the Cellular Thermal Shift Assay (CETSA). Cells (2019) 8:1275. doi: 10.3390/cells8101275
94. Lin M-H, Lee Y-H, Cheng H-L, Chen H-Y, Jhuang F-H, Chueh PJ. Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (tNOX) and Sirtuin1 (SIRT1). Molecules (2016) 21:849. doi: 10.3390/molecules21070849
95. Jin J, Lin G, Huang H, Xu D, Yu H, Ma X, et al. Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating P53. Int J Biol Sci (2014) 10:285. doi: 10.7150/ijbs.7730
96. Li Q, Wang XH, Yang ZH, Wang HP, Yang ZW, Li SW, et al. Induction of Cell Cycle Arrest in Bladder Cancer RT4 Cells by Capsaicin. Zhonghua Yi Xue Za Zhi (2010) 90:1230–3.
97. Wong RS. Apoptosis in Cancer: From Pathogenesis to Treatment. J Exp Clin Cancer Res (2011) 30:87. doi: 10.1186/1756-9966-30-87
98. Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, et al. Capsaicin-Induced Apoptosis of Glioma Cells Is Mediated by TRPV1 Vanilloid Receptor and Requires P38 MAPK Activation. J Neurochem (2007) 102:977–90. doi: 10.1111/j.1471-4159.2007.04582.x
99. Xu S, Cheng X, Wu L, Zheng J, Wang X, Wu J, et al. Capsaicin Induces Mitochondrial Dysfunction and Apoptosis in Anaplastic Thyroid Carcinoma Cells via TRPV1-Mediated Mitochondrial Calcium Overload. Cell Signal (2020) 75:109733. doi: 10.1016/j.cellsig.2020.109733
100. Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of Transient Receptor Potential Vanilloid Type 1 (TRPV1) by Capsaicin Induces Fas/CD95-Mediated Apoptosis of Urothelial Cancer Cells in an ATM-Dependent Manner. Carcinogenesis (2009) 30:1320–9. doi: 10.1093/carcin/bgp138
101. Hou N, He X, Yang Y, Fu J, Zhang W, Guo Z, et al. TRPV1 Induced Apoptosis of Colorectal Cancer Cells by Activating Calcineurin-NFAT2-P53 Signaling Pathway. BioMed Res Int (2019) 2019:1–8. doi: 10.1155/2019/6712536
102. Lau JK, Brown KC, Dom AM, Witte TR, Thornhill BA, Crabtree CM, et al. Capsaicin Induces Apoptosis in Human Small Cell Lung Cancer via the TRPV6 Receptor and the Calpain Pathway. Apoptosis (2014) 19:1190–201. doi: 10.1007/s10495-014-1007-y
103. Chow J, Norng M, Zhang J, Chai J. TRPV6 Mediates Capsaicin-Induced Apoptosis in Gastric Cancer Cells—Mechanisms Behind a Possible New “Hot” Cancer Treatment. Biochim Biophys Acta (BBA)-Molecular Cell Res (2007) 1773:565–76. doi: 10.1016/j.bbamcr.2007.01.001
104. Ferreira LGB, Faria JV, Dos Santos JPS, Faria RX. Capsaicin: TRPV1-Independent Mechanisms and Novel Therapeutic Possibilities. Eur J Pharmacol (2020) 887:173356. doi: 10.1016/j.ejphar.2020.173356
105. Bao Z, Dai X, Wang P, Tao Y, Chai D. Capsaicin Induces Cytotoxicity in Human Osteosarcoma MG63 Cells Through TRPV1-Dependent and-Independent Pathways. Cell Cycle (2019) 18:1379–92. doi: 10.1080/15384101.2019.1618119
106. Kida R, Noguchi T, Murakami M, Hashimoto O, Kawada T, Matsui T, et al. Supra-Pharmacological Concentration of Capsaicin Stimulates Brown Adipogenesis Through Induction of Endoplasmic Reticulum Stress. Sci Rep (2018) 8:1–14. doi: 10.1038/s41598-018-19223-2
107. Pramanik KC, Boreddy SR, Srivastava SK. Role of Mitochondrial Electron Transport Chain Complexes in Capsaicin Mediated Oxidative Stress Leading to Apoptosis in Pancreatic Cancer Cells. PloS One (2011) 6:e20151. doi: 10.1371/journal.pone.0020151
108. Zhang R, Humphreys I, Sahu RP, Shi Y, Srivastava SK. In Vitro and In Vivo Induction of Apoptosis by Capsaicin in Pancreatic Cancer Cells is Mediated Through ROS Generation and Mitochondrial Death Pathway. Apoptosis (2008) 13:1465–78. doi: 10.1007/s10495-008-0278-6
109. Denton D, Kumar S. Autophagy-Dependent Cell Death. Cell Death Differ (2019) 26:605–16. doi: 10.1038/s41418-018-0252-y
110. Liu Y-P, Dong F-X, Chai X, Zhu S, Zhang B-L, Gao D-S. Role of Autophagy in Capsaicin-Induced Apoptosis in U251 Glioma Cells. Cell Mol Neurobiol (2016) 36:737–43. doi: 10.1007/s10571-015-0254-y
111. Chu H, Li M, Wang X. Capsaicin Induces Apoptosis and Autophagy in Human Melanoma Cells. Oncol Lett (2019) 17:4827–34. doi: 10.3892/ol.2019.10206
112. Lin Y-T, Wang H-C, Hsu Y-C, Cho C-L, Yang M-Y, Chien C-Y. Capsaicin Induces Autophagy and Apoptosis in Human Nasopharyngeal Carcinoma Cells by Downregulating the PI3K/AKT/mTOR Pathway. Int J Mol Sci (2017) 18:1343. doi: 10.3390/ijms18071343
113. Islam A, Hsieh P-F, Liu P-F, Chou J-C, Liao J-W, Hsieh M-K, et al. Capsaicin Exerts Therapeutic Effects by Targeting tNOX-SIRT1 Axis and Augmenting ROS-Dependent Autophagy in Melanoma Cancer Cells. Am J Cancer Res (2021) 11:4199.
114. Chang C-F, Islam A, Liu P-F, Zhan J-H, Chueh PJ. Capsaicin Acts Through tNOX (ENOX2) to Induce Autophagic Apoptosis in P53-Mutated HSC-3 Cells But Autophagy in P53-Functional SAS Oral Cancer Cells. Am J Cancer Res (2020) 10:3230.
115. Chen X, Tan M, Xie Z, Feng B, Zhao Z, Yang K, et al. Inhibiting ROS-STAT3-Dependent Autophagy Enhanced Capsaicin–Induced Apoptosis in Human Hepatocellular Carcinoma Cells. Free Radic Res (2016) 50:744–55. doi: 10.3109/10715762.2016.1173689
116. Oh S-H, Lim S-C. Endoplasmic Reticulum Stress-Mediated Autophagy/Apoptosis Induced by Capsaicin (8-Methyl-N-Vanillyl-6-Nonenamide) and Dihydrocapsaicin Is Regulated by the Extent of C-Jun NH2-Terminal Kinase/Extracellular Signal-Regulated Kinase Activation in WI38 Lung Ep. J Pharmacol Exp Ther (2009) 329:112–22. doi: 10.1124/jpet.108.144113
117. Choi C-H, Jung Y-K, Oh S-H. Autophagy Induction by Capsaicin in Malignant Human Breast Cells Is Modulated by P38 and Extracellular Signal-Regulated Mitogen-Activated Protein Kinases and Retards Cell Death by Suppressing Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol Pharmacol (2010) 78:114–25. doi: 10.1124/mol.110.063495
118. Amantini C, Mosca M, Lucciarini R, Perfumi M, Morrone S, Piccoli M, et al. Distinct Thymocyte Subsets Express the Vanilloid Receptor VR1 That Mediates Capsaicin-Induced Apoptotic Cell Death. Cell Death Differ (2004) 11:1342–56. doi: 10.1038/sj.cdd.4401506
119. Jambrina E, Alonso R, Alcalde M, del Carmen Rodrıguez M, Serrano A, Martı́nez AC, et al. Calcium Influx Through Receptor-Operated Channel Induces Mitochondria-Triggered Paraptotic Cell Death. J Biol Chem (2003) 278:14134–45. doi: 10.1074/jbc.M211388200
120. Amantini C, Morelli MB, Nabissi M, Cardinali C, Santoni M, Gismondi A, et al. Capsaicin Triggers Autophagic Cell Survival Which Drives Epithelial Mesenchymal Transition and Chemoresistance in Bladder Cancer Cells in an Hedgehog-Dependent Manner. Oncotarget (2016) 7:50180. doi: 10.18632/oncotarget.10326
121. Wu TTL, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR. Consequences of Activating the Calcium-Permeable Ion Channel TRPV1 in Breast Cancer Cells With Regulated TRPV1 Expression. Cell Calcium (2014) 56:59–67. doi: 10.1016/j.ceca.2014.04.006
122. Ramírez-Barrantes R, Córdova C, Gatica S, Rodriguez B, Lozano C, Marchant I, et al. Transient Receptor Potential Vanilloid 1 Expression Mediates Capsaicin-Induced Cell Death. Front Physiol (2018) 9:682. doi: 10.3389/fphys.2018.00682
123. Huang Y-C, Yuan T-M, Liu B-H, Liu K-L, Wung C-H, Chuang S-M. Capsaicin Potentiates Anticancer Drug Efficacy Through Autophagy-Mediated Ribophorin II Downregulation and Necroptosis in Oral Squamous Cell Carcinoma Cells. Front Pharmacol (2021) 12:676813. doi: 10.3389/fphar.2021.676813
124. Dillekås H, Rogers MS, Straume O. Are 90% of Deaths From Cancer Caused by Metastases? Cancer Med (2019) 8:5574–6. doi: 10.1002/cam4.2474
125. Min J-K, Han K-Y, Kim E-C, Kim Y-M, Lee S-W, Kim O-H, et al. Capsaicin Inhibits In Vitro and In Vivo Angiogenesis. Cancer Res (2004) 64:644–51. doi: 10.1158/0008-5472.CAN-03-3250
126. Chakraborty S, Adhikary A, Mazumdar M, Mukherjee S, Bhattacharjee P, Guha D, et al. Capsaicin-Induced Activation of P53-SMAR1 Auto-Regulatory Loop Down-Regulates VEGF in non-Small Cell Lung Cancer to Restrain Angiogenesis. PloS One (2014) 9:e99743. doi: 10.1371/journal.pone.0099743
127. Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, et al. Suppression of EGF-Induced Tumor Cell Migration and Matrix Metalloproteinase-9 Expression by Capsaicin via the Inhibition of EGFR-Mediated FAK/Akt, PKC/Raf/ERK, P38 MAPK, and AP-1 Signaling. Mol Nutr Food Res (2011) 55:594–605. doi: 10.1002/mnfr.201000292
128. Lee G-R, Jang SH, Kim CJ, Kim A-R, Yoon D-J, Park N-H, et al. Capsaicin Suppresses the Migration of Cholangiocarcinoma Cells by Down-Regulating Matrix Metalloproteinase-9 Expression via the AMPK-NF-κb Signaling Pathway. Clin Exp Metastasis (2014) 31:897–907. doi: 10.1007/s10585-014-9678-x
129. Shin DH, Kim OH, Jun HS, Kang MK. Inhibitory Effect of Capsaicin on B16-F10 Melanoma Cell Migration via the Phosphatidylinositol 3-Kinase/Akt/Rac1 Signal Pathway. Exp Mol Med (2008) 40:486–94. doi: 10.3858/emm.2008.40.5.486
130. Xu S, Zhang L, Cheng X, Yu H, Bao J, Lu R. Capsaicin Inhibits the Metastasis of Human Papillary Thyroid Carcinoma BCPAP Cells Through the Modulation of the TRPV1 Channel. Food Funct (2018) 9:344–54. doi: 10.1039/C7FO01295K
131. Feng J, Xu Y, Wei Z, Xia Y, Zhang H, Shen C, et al. Capsaicin Inhibits Migration and Invasion via Inhibiting Epithelial-Mesenchymal Transition in Esophageal Squamous Cell Carcinoma by Up-Regulation of Claudin-3 Expression. J Funct Foods (2022) 89:104934. doi: 10.1016/j.jff.2022.104934
132. Venier NA, Yamamoto T, Sugar LM, Adomat H, Fleshner NE, Klotz LH, et al. Capsaicin Reduces the Metastatic Burden in the Transgenic Adenocarcinoma of the Mouse Prostate Model. Prostate (2015) 75:1300–11. doi: 10.1002/pros.23013
133. Privitera R, Anand P. Capsaicin 8% Patch Qutenza and Other Current Treatments for Neuropathic Pain in Chemotherapy-Induced Peripheral Neuropathy (CIPN). Curr Opin Support Palliat Care (2021) 15:125–31. doi: 10.1097/SPC.0000000000000545
134. Remadevi R, Szallisi A. Adlea (ALGRX-4975), an Injectable Capsaicin (TRPV1 Receptor Agonist) Formulation for Longlasting Pain Relief. IDrugs (2008) 11:120–32.
135. Okuno SH, Foote RL, Olmscheid MA, Sloan J, Sapiente RA, Novotny PJ. Evaluation of an Oral Capsaicin Lozenge for Preventing Radiation-Induced Mucositis. J Cancer Integr Med (2004) 2:179–83.
136. Bode AM, Dong Z. The Two Faces of Capsaicin. Cancer Res (2011) 71:2809–14. doi: 10.1158/0008-5472.CAN-10-3756
137. Friedman JR, Nolan NA, Brown KC, Miles SL, Akers AT, Colclough KW, et al. Anticancer Activity of Natural and Synthetic Capsaicin Analogs. J Pharmacol Exp Ther (2018) 364:462–73. doi: 10.1124/jpet.117.243691
138. Chanda S, Mould A, Esmail A, Bley K. Toxicity Studies With Pure Trans-Capsaicin Delivered to Dogs via Intravenous Administration. Regul Toxicol Pharmacol (2005) 43:66–75. doi: 10.1016/j.yrtph.2005.06.003
139. Lewinska A, Chochrek P, Smolag K, Rawska E, Wnuk M. Oxidant-Based Anticancer Activity of a Novel Synthetic Analogue of Capsaicin, Capsaicin Epoxide. Redox Rep (2015) 20:116–25. doi: 10.1179/1351000214Y.0000000113
140. Pereira GJV, Tavares MT, Azevedo RA, Martins BB, Cunha MR, Bhardwaj R, et al. Capsaicin-Like Analogue Induced Selective Apoptosis in A2058 Melanoma Cells: Design, Synthesis and Molecular Modeling. Bioorg Med Chem (2019) 27:2893–904. doi: 10.1016/j.bmc.2019.05.020
141. de-Sá-Júnior PL, Pasqualoto KFM, Ferreira AK, Tavares MT, Damião MCFCB, de Azevedo RA, et al. RPF101, a New Capsaicin-Like Analogue, Disrupts the Microtubule Network Accompanied by Arrest in the G2/M Phase, Inducing Apoptosis and Mitotic Catastrophe in the MCF-7 Breast Cancer Cells. Toxicol Appl Pharmacol (2013) 266:385–98. doi: 10.1016/j.taap.2012.11.029
142. Yang MH, Jung SH, Sethi G, Ahn KS. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, Against Various Cancers and Inflammatory Diseases. Molecules (2019) 24:995. doi: 10.3390/molecules24050995
143. Chittepu VCSR, Kalhotra P, Revilla GIO, Velázquez TG. Emerging Technologies to Improve Capsaicin Delivery and Its Therapeutic Efficacy. Capsaicin its Hum Ther Dev IntechOpen (2018).
144. Feng Y, Zhu Y, Wan J, Yang X, Firempong CK, Yu J, et al. Enhanced Oral Bioavailability, Reduced Irritation and Increased Hypolipidemic Activity of Self-Assembled Capsaicin Prodrug Nanoparticles. J Funct Foods (2018) 44:137–45. doi: 10.1016/j.jff.2018.03.006
145. De Freitas GBL, De Almeida DJ, Carraro E, Kerppers II, Martins GAG, Mainardes RM, et al. Formulation, Characterization, and In Vitro/In Vivo Studies of Capsaicin-Loaded Albumin Nanoparticles. Mater Sci Eng C (2018) 93:70–9. doi: 10.1016/j.msec.2018.07.064
146. Kunjiappan S, Sankaranarayanan M, Kumar BK, Pavadai P, Babkiewicz E, Maszczyk P, et al. Capsaicin-Loaded Solid Lipid Nanoparticles: Design, Biodistribution, in Silico Modeling and In Vitro Cytotoxicity Evaluation. Nanotechnology (2020) 32:95101. doi: 10.1088/1361-6528/abc57e
147. Lv L, Zhuang Y, Zhang H, Tian N, Dang W, Wu S. Capsaicin-Loaded Folic Acid-Conjugated Lipid Nanoparticles for Enhanced Therapeutic Efficacy in Ovarian Cancers. BioMed Pharmacother (2017) 91:999–1005. doi: 10.1016/j.biopha.2017.04.097
148. Yu T, Tong L, Ao Y, Zhang G, Liu Y, Zhang H. Novel Design of NIR-Triggered Plasmonic Nanodots Capped Mesoporous Silica Nanoparticles Loaded With Natural Capsaicin to Inhibition of Metastasis of Human Papillary Thyroid Carcinoma B-CPAP Cells in Thyroid Cancer Chemo-Photothermal Therapy. J Photochem Photobiol B Biol (2019) 197:111534. doi: 10.1016/j.jphotobiol.2019.111534
149. Hazem NM, ElKashef WF, El-Sherbiny IM, Emam AA, Shaalan D, Sobh M. Anticarcinogenic Effects of Capsaicin-Loaded Nanoparticles on In Vitro Hepatocellular Carcinoma. Curr Chem Biol (2021) 15:188–201. doi: 10.2174/2212796814999201116211648
150. Parashar P, Tripathi CB, Arya M, Kanoujia J, Singh M, Yadav A, et al. A Facile Approach for Fabricating CD44-Targeted Delivery of Hyaluronic Acid-Functionalized PCL Nanoparticles in Urethane-Induced Lung Cancer: Bcl-2, MMP-9, Caspase-9, and BAX as Potential Markers. Drug Deliv Transl Res (2019) 9:37–52. doi: 10.1007/s13346-020-00783-8
151. Al-Samydai A, Alshaer W, Al-Dujaili EAS, Azzam H, Aburjai T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients (2021) 13:3995. doi: 10.3390/nu13113995
152. Abdelnabi H, Alshaer W, Azzam H, Alqudah D, Al-Samydai A, Aburjai T. Loading of Capsaicin-in-Cyclodextrin Inclusion Complexes Into PEGylated Liposomes and the Inhibitory Effect on IL-8 Production by MDA-MB-231 and A549 Cancer Cell Lines. Z für Naturforsch C (2021) 76:503–14. doi: 10.1515/znc-2021-0018
153. Sampedro A, Ramos-Torres Á, Schwöppe C, Mück-Lichtenfeld C, Helmers I, Bort A, et al. Hierarchical Self-Assembly of BODIPY Dyes as a Tool to Improve the Antitumor Activity of Capsaicin in Prostate Cancer. Angew Chemie Int Ed (2018) 57:17235–9. doi: 10.1002/anie.201804783
154. Lu M, Chen C, Lan Y, Xiao J, Li R, Huang J, et al. Capsaicin—the Major Bioactive Ingredient of Chili Peppers: Bio-Efficacy and Delivery Systems. Food Funct (2020) 11:2848–60. doi: 10.1039/D0FO00351D
155. von Palubitzki L, Wang Y, Hoffmann S, Vidal-y-Sy S, Zobiak B, Failla AV, et al. Differences of the Tumour Cell Glycocalyx Affect Binding of Capsaicin-Loaded Chitosan Nanocapsules. Sci Rep (2020) 10:1–16. doi: 10.1038/s41598-020-79882-y
156. Amruthraj NJ, Preetam Raj JP, Lebel A. Capsaicin-Capped Silver Nanoparticles: Its Kinetics, Characterization and Biocompatibility Assay. Appl Nanosci (2015) 5:403–9. doi: 10.1007/s13204-014-0330-5
157. Parashar P, Tripathi CB, Arya M, Kanoujia J, Singh M, Yadav A, et al. A Synergistic Approach for Management of Lung Carcinoma Through Folic Acid Functionalized Co-Therapy of Capsaicin and Gefitinib Nanoparticles: Enhanced Apoptosis and Metalloproteinase-9 Down-Regulation. Phytomedicine (2019) 53:107–23. doi: 10.1016/j.phymed.2018.09.013
158. Hong Z-F, Zhao W-X, Yin Z-Y, Xie C-R, Xu Y-P, Chi X-Q, et al. Capsaicin Enhances the Drug Sensitivity of Cholangiocarcinoma Through the Inhibition of Chemotherapeutic-Induced Autophagy. PloS One (2015) 10:e0121538. doi: 10.1371/journal.pone.0121538
159. Kim S, Oh EY, Lee JH, Nam D, Lee SG, Lee J, et al. Brassinin Combined With Capsaicin Enhances Apoptotic and Anti-Metastatic Effects in PC-3 Human Prostate Cancer Cells. Phyther Res (2015) 29:1828–36. doi: 10.1002/ptr.5478
160. Lan Y, Sun Y, Yang T, Ma X, Cao M, Liu L, et al. Co-Delivery of Paclitaxel by a Capsaicin Prodrug Micelle Facilitating for Combination Therapy on Breast Cancer. Mol Pharm (2019) 16:3430–40. doi: 10.1021/acs.molpharmaceut.9b00209
161. Wang Y, Deng X, Yu C, Zhao G, Zhou J, Zhang G, et al. Synergistic Inhibitory Effects of Capsaicin Combined With Cisplatin on Human Osteosarcoma in Culture and in Xenografts. J Exp Clin Cancer Res (2018) 37:251. doi: 10.1186/s13046-018-0922-0
162. Hwang J, Lee Y, Shin J, Park OJ. Anti-Inflammatory and Anticarcinogenic Effect of Genistein Alone or in Combination With Capsaicin in TPA-Treated Rat Mammary Glands or Mammary Cancer Cell Line. Ann N Y Acad Sci (2009) 1171:415–20. doi: 10.1111/j.1749-6632.2009.04696.x
163. Sánchez BG, Bort A, Mateos-Gómez PA, Rodríguez-Henche N, Díaz-Laviada I. Combination of the Natural Product Capsaicin and Docetaxel Synergistically Kills Human Prostate Cancer Cells Through the Metabolic Regulator AMP-Activated Kinase. Cancer Cell Int (2019) 19:1–14. doi: 10.1186/s12935-019-0769-2
164. Qi C, Wang D, Gong X, Zhou Q, Yue X, Li C, et al. Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. ACS Appl Mater Interfaces (2021) 13:16019–35. doi: 10.1021/acsami.0c23137
165. Zhang S, Ni Y, Zhao C, Qiao Z, Yu H, Wang L, et al. Capsaicin Enhances the Antitumor Activity of Sorafenib in Hepatocellular Carcinoma Cells and Mouse Xenograft Tumors Through Increased ERK Signaling. Acta Pharmacol Sin (2018) 39:438–48. doi: 10.1038/aps.2017.156
166. Vendrely V, Amintas S, Noel C, Moranvillier I, Lamrissi I, Rousseau B, et al. Combination Treatment of Resveratrol and Capsaicin Radiosensitizes Pancreatic Tumor Cells by Unbalancing DNA Repair Response to Radiotherapy Towards Cell Death. Cancer Lett (2019) 451:1–10. doi: 10.1016/j.canlet.2019.02.038
167. Bort A, Spínola E, Rodríguez-Henche N, Díaz-Laviada I. Capsaicin Exerts Synergistic Antitumor Effect With Sorafenib in Hepatocellular Carcinoma Cells Through AMPK Activation. Oncotarget (2017) 8:87684–98. doi: 10.18632/oncotarget.21196
168. Clark R, Lee J, Lee S-H. Synergistic Anticancer Activity of Capsaicin and 3,3′-Diindolylmethane in Human Colorectal Cancer. J Agric Food Chem (2015) 63:4297–304. doi: 10.1021/jf506098s
169. Li H, Krstin S, Wang S, Wink M. Capsaicin and Piperine can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Mol (2018) 23:557. doi: 10.3390/molecules23030557
170. Zheng L, Chen J, Ma Z, Liu W, Yang F, Yang Z, et al. Capsaicin Enhances Anti-Proliferation Efficacy of Pirarubicin via Activating TRPV1 and Inhibiting PCNA Nuclear Translocation in 5637 Cells. Mol Med Rep (2016) 13:881–7. doi: 10.3892/mmr.2015.4623
171. Li H, Li F, Sun Y, Li Y. A Feasible Strategy of Fabricating Hybrid Drugs Encapsulated Polymeric Nanoparticles for the Treatment of Gastric Cancer Therapy. Process Biochem (2021) 109:19–26. doi: 10.1016/j.procbio.2021.06.001
172. Venier NA, Colquhoun AJ, Sasaki H, Kiss A, Sugar L, Adomat H, et al. Capsaicin: A Novel Radio-Sensitizing Agent for Prostate Cancer. Prostate (2015) 75:113–25. doi: 10.1002/pros.22896
173. Chen J-C, Ko J-C, Yen T-C, Chen T-Y, Lin Y-C, Ma P-F, et al. Capsaicin Enhances Erlotinib-Induced Cytotoxicity via AKT Inactivation and Excision Repair Cross-Complementary 1 (ERCC1) Down-Regulation in Human Lung Cancer Cells. Toxicol Res (Camb) (2019) 8:459–70. doi: 10.1039/C8TX00346G
Keywords: anticancer, bibliometrics, cytotoxicity, TRPV 1, vanilloid
Citation: Adetunji TL, Olawale F, Olisah C, Adetunji AE and Aremu AO (2022) Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 12:908487. doi: 10.3389/fonc.2022.908487
Received: 30 March 2022; Accepted: 30 May 2022;
Published: 13 July 2022.
Edited by:
Subrata Kumar Pore, Amity University, IndiaReviewed by:
Sujan K. Mondal, University of Pittsburgh, United StatesCopyright © 2022 Adetunji, Olawale, Olisah, Adetunji and Aremu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ademola Emmanuel Adetunji, YWRldHVuamlhZGVtb2xhQGhvdG1haWwuY29t; Adeyemi Oladapo Aremu, YXJlbXVhQHVrem4uYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.