- 1Emergency Medicine Clinical Research Center, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Clinical Center for Medicine in Acute Infection, Capital Medical University, Beijing, China
Objective: To analyze the impact of different methods of Vitamin D administration on the prognosis of COVID-19 patients.
Methods: A comprehensive literature search was conducted across four databases: PubMed, Embase, Web of Science, and Cochrane, up to January 5, 2024. Eligible studies included randomized controlled trials and cohort studies that compared Vitamin D supplementation with control groups in COVID-19 patients. Outcomes of interest were mortality rate, ICU (Intensive Care Unit) admission rate, length of hospital stay, and endotracheal intubation rate. Subgroup analyses were performed based on the dosing regimen (single-dose vs. continuous-dose), total Vitamin D intake within 14 days (≥100,000 IU vs. <100,000 IU), and baseline serum Vitamin D levels (deficient group: 25OHD < 30 ng/mL vs. non-restricted group). A random-effects model was employed for meta-analysis to account for heterogeneity among studies.
Results: A total of 21 studies involving 4,553 participants were included. In terms of mortality, Vitamin D supplementation significantly reduced the mortality rate (RR = 0.72, 95% CI: 0.54–0.94, I2 = 54%, p = 0.02), with continuous dosing being more effective (RR = 0.53, 95% CI: 0.34–0.83, I2 = 55%, p = 0.006) compared to single-dose (RR = 0.88, 95% CI: 0.69–1.12, I2 = 21%, p = 0.3), and lower total doses (<100,000 IU) showing greater benefit (RR = 0.30, 95% CI: 0.21–0.44, I2 = 0%, p < 0.0001). Mortality was significantly reduced in the Vitamin D-deficient group (25OHD < 30 ng/mL) (RR = 0.73, 95% CI: 0.59–0.89, I2 = 0%, p = 0.002) but not in the non-restricted group. Regarding ICU admission, supplementation reduced ICU admission rates (RR = 0.58, 95% CI: 0.38–0.88, I2 = 74%, p = 0.01), with continuous dosing (RR = 0.44, 95% CI: 0.22–0.90, I2 = 74%, p = 0.02) being more effective than single-dose (RR = 0.79, 95% CI: 0.61–1.03, I2 = 22%, p = 0.08), and lower doses (<100,000 IU) providing more significant reduction (RR = 0.31, 95% CI: 0.21–0.47, I2 = 0%, p = 0.001). ICU admission rates were significantly reduced in the Vitamin D-deficient group (RR = 0.63, 95% CI: 0.42–0.93, I2 = 0%, p = 0.02) but not in the non-restricted group (RR = 0.59, 95% CI: 0.32–1.11, I2 = 86%, p = 0.1). For length of hospital stay, no significant differences were observed between Vitamin D and control groups (MD = −1, 95% CI: −2.16 to 0.16, p = 0.13), and subgroup analyses by dosing regimen, total dose, and baseline Vitamin D levels also showed no significant differences. Similarly, for endotracheal intubation, there was no significant difference in intubation rates between groups (RR = 0.78, 95% CI: 0.56–1.08, p = 0.13), and subgroup analyses confirmed no significant effect of different dosing strategies or baseline Vitamin D status on intubation rates.
Conclusion: Vitamin D supplementation improves clinical outcomes in COVID-19 patients by reducing mortality and ICU admission rates, particularly when administered continuously with a total dose of less than 100,000 IU over 14 days, and among those with baseline Vitamin D deficiency (25OHD < 30 ng/mL). However, there were no significant effects on the length of hospital stay or endotracheal intubation rates, regardless of the dosing regimen or baseline Vitamin D levels. These findings emphasize the importance of considering both the total dose over 14 days and baseline Vitamin D status to optimize therapeutic benefits.
1 Introduction
Coronavirus Disease 2019 (COVID-19) is a systemic respiratory disease caused by the novel coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2:SARS-CoV-2). Since December 2019, COVID-19 has spread globally, affecting millions of people and resulting in hundreds of thousands of deaths. Significant progress has been made in the prevention and treatment of COVID-19 using effective vaccines and antiviral drugs. However, intermittent outbreaks of the novel coronavirus continue worldwide, posing a threat to human health and life. Thus, there is a need to explore effective preventive and therapeutic drugs to aid epidemic control. Previous studies have stated that Vitamin D enhances innate and cellular immunity (1–3) and reduces the survival and replication of respiratory viruses. Besides, numerous studies have established an association between low Vitamin D levels and an increased risk of acute respiratory virus infections (4, 5), which can be reduced through Vitamin D supplementation (6, 7). Moreover, Vitamin D supplementation has also been associated with a reduction in all-cause mortality (8). Since the COVID-19 pandemic began, some studies and meta-analyses have confirmed an association between low blood Vitamin D levels and adverse outcomes in patients with novel coronavirus infection (9–13). Nevertheless, research results regarding using Vitamin D supplementation to improve outcomes in patients with COVID-19 infection are not clear. Previous systematic reviews and meta-analyses of RCTs (randomized controlled trials) have yielded inconsistent results. Some RCT studies have asserted that supplementation with Vitamin D shortens the recovery time for mild to moderate COVID-19 symptoms such as cough and loss of taste (14). Furthermore, there have been reports of reduced severity (length of hospital stay, need for oxygen or respiratory support, etc.) and mortality rates (15–19). However, these findings are inconsistent, since other studies have concluded that supplementation with Vitamin D does not improve mortality rates or any other severity indicators in COVID-19 patients, including the need for endotracheal intubation and length of hospital stay (20–24).
By pooling results from diverse studies, we aim to provide a clearer understanding of the overall impact of Vitamin D supplementation on COVID-19 outcomes. This approach not only enhances the statistical power and generalizability of our findings but also helps to identify patterns and factors that may explain the variability in individual study results. Specifically, this review examines whether immediate Vitamin D supplementation upon hospital admission can improve the prognosis of COVID-19 patients. By comparing the outcomes across various target populations, dosages, and methods of Vitamin D supplementation, we aim to identify the most effective strategies for administering Vitamin D to these patients.
2 Research methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25) and is registered in PROSPERO website: https://www.crd.york.ac.uk/prospero/ID:CRD42024545945, PROSPERO Registration No: CRD42024545945.
2.1 Search strategy
Searches were conducted in the PubMed, Embase, Web of Science, and Cochrane databases, with search dates ranging from inception to January 5, 2024. The search strategy followed the PICOS principles, primarily focusing on the study population, intervention methods, and research methodology. The search terms and keywords used were: “COVID-19,” “2019-nCoV Infection,” “infection 2019-nCoV,” “SARS-CoV-2 Infection,” “SARS CoV 2 Infection,” “2019 Novel Coronavirus Disease,” “2019 Novel Coronavirus Infection,” “COVID-19 Virus Infections,” “Infection COVID-19 Virus,” “Virus Infection COVID-19,” “Coronavirus Disease 2019,” “Disease 2019, Coronavirus,” “Coronavirus Disease 19,” “Severe Acute Respiratory Syndrome Coronavirus 2 Infection,” “COVID-19 Virus Disease,” “Disease COVID-19 Virus,” “Virus Disease, COVID-19,” “SARS Coronavirus 2 Infection,” “2019 nCoV Disease,” “Disease, 2019-nCoV,” “COVID-19 Pandemic,” “Pandemic, COVID-19,” “Vitamin D,” “Calciol,” “Vitamin D 3,” “Cholecalciferol,” “25 HydroxyVitamin D3,” “Calcidiol,” “25 Hydroxycholecalciferol,” “Calcifediol,” “Dedrogyl,” “Hydropherol,” and “Calderol.” The specific search strings are provided in Supplementary Table S1. To supplement the research, manual searches were conducted by retrieving bibliographies of relevant reviews and identified articles. If necessary, contact was made with the study authors to obtain additional information.
2.2 Inclusion and exclusion criteria
The inclusion criteria for this meta-analysis were based on the following Population, Intervention, Comparison, Outcomes, and Study design (PICOS) criteria: (1) Participants: admitted patients aged ≥18 years with confirmed COVID-19 diagnosis; (2) Intervention: supplementation with Vitamin D; (3) Comparison: no Vitamin D supplementation or lower-dose supplementation; (4) Outcome: mortality, ICU admission, length of hospital stay, or need for endotracheal intubation; (5) Study design: randomized controlled trials or observational studies. Certain articles including reviews, simulation studies, animal studies, letters, conference papers, and case studies were all excluded from this study.
2.3 Data extraction
Data extraction was performed independently by two researchers (Yixuan LI and Ying Zhang). Any discrepancies were resolved by referring to the third author, Zhang Xiangqun, if necessary. The following information was extracted from all eligible studies: first author’s name, publication year, country of study, study design, patient characteristics, intervention methods and dosage, and clinical outcomes (mortality rate, ICU admission rate, length of hospital stay, and rate of endotracheal intubation). Data from included studies were entered into a dedicated spreadsheet using Microsoft Excel (Microsoft Corporation, Redmond, WA, United States). In cases of missing data regarding the primary outcomes, we contacted the corresponding authors of the original studies.
2.4 Statistical analysis
All analyses were conducted using Review Manager software version 5.4 (Nordic Cochrane Center, Cochrane Collaboration). A significance level of p < 0.05 (two-tailed) was used for all statistical tests. For binary data, risk ratios (OR) and their 95% confidence intervals (CI) were calculated. For continuous data, mean differences (MD) and their 95% CIs were calculated.
In assessing heterogeneity, we selected the model based on its extent. A fixed-effects model was employed when the variability across studies was minimal, suggesting similar effect sizes. Conversely, a random-effects model was used when substantial variability was present, reflecting diverse effect sizes. This approach not only considers the degree of heterogeneity but also accounts for study characteristics such as design, sample demographics, intervention specifics, outcome measurement methods, and contextual factors. By integrating these considerations, we aimed to accurately reflect both within-study and between-study variations, ensuring a robust and reliable overall effect estimate.
If the effect size was represented by standardized mean differences (MD) along with 95% CIs, the mean (26) and standard deviation (27) of the quartile data were calculated using the provided formulas. Publication bias for each study was assessed by constructing funnel plots of the effect size against standard error. Sensitivity analysis was conducted by systematically excluding individual studies to assess the robustness of the results. Subgroup treatment effects were compared using Cochran’s Q test and Higgins’s I2 statistic, with p < 0.05 indicating a statistically significant difference.
2.5 Assessment of study quality
The quality of the included literature was assessed by author Hongmeng Dong and Na Shang according to the quality assessment criteria of the Cochrane Collaboration (28). When including randomized controlled trials, the Cochrane Handbook recommends using a revised version of the Cochrane tool, known as the Risk of Bias tool (RoB 2) (29). The RoB 2 tool provides a framework for assessing the risk of bias for individual outcomes in any type of randomized trial. Evaluation criteria include random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other biases. In this study, the reviewers assessed different studies based on the Cochrane Handbook guidelines. The risk of bias for each domain can be categorized into three levels: “low risk,” “some concerns,” and “high risk.” If all domains are assessed as low risk, the overall risk of bias is low. If one or more domains are judged as “some concerns” but none are assessed as high risk, the overall risk of bias is “some concerns.” If at least one domain is evaluated as high risk, the overall risk of bias is considered “high risk” (30).
3 Results
3.1 Study selection
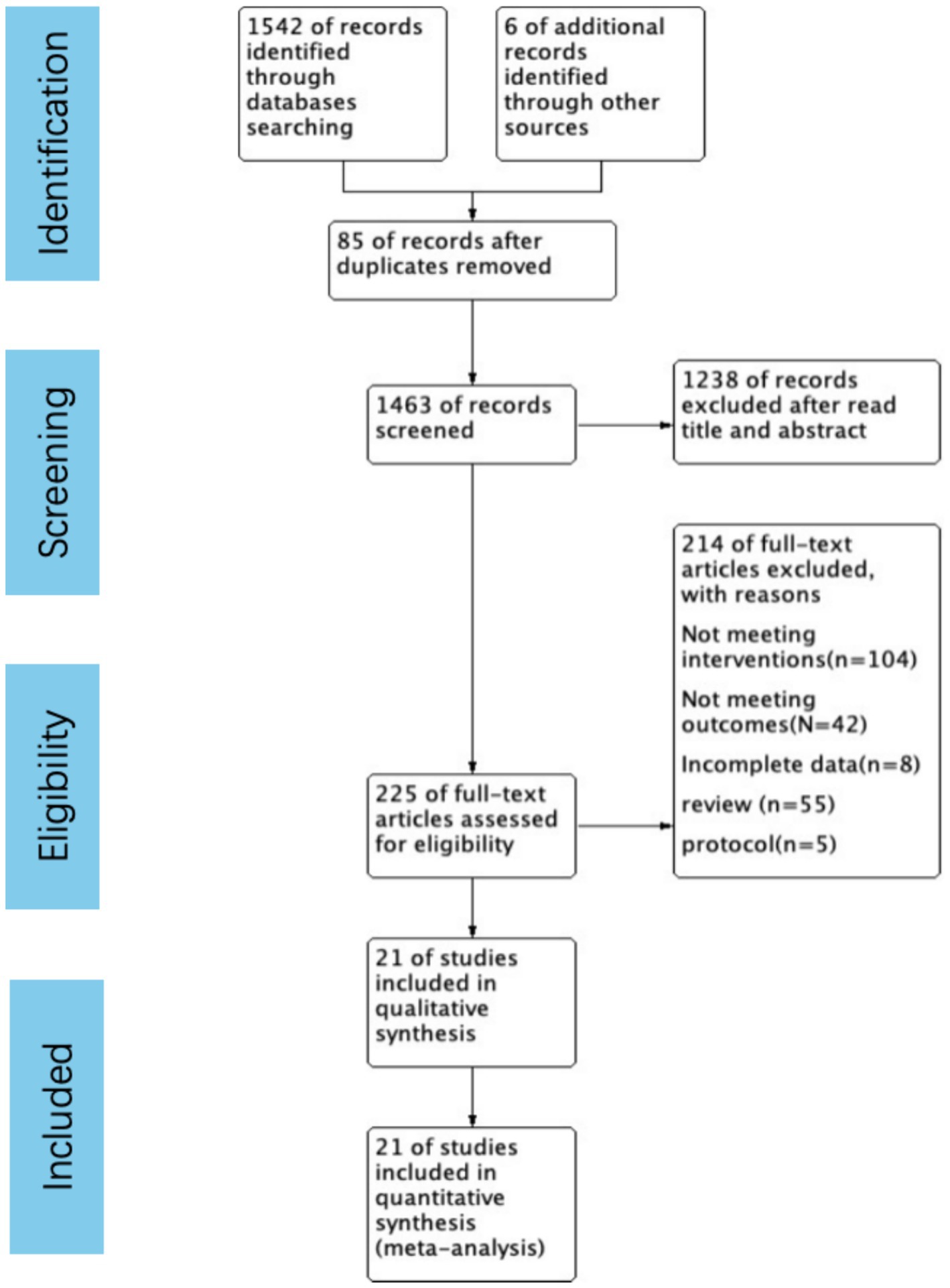
The systematic review, selection, and exclusion criteria are summarized in Figure 1. A total of 1,548 articles were retrieved, and after removing duplicate publications, 1,463 articles were included for analysis. After carefully reviewing the titles and abstracts, 225 articles remained. The full texts were all read thoroughly, and then a final selection of 21 articles (14, 16–21, 23, 24, 31–42) was made. These studies included a total of 4,553 patients, with 2,164 patients in the Vitamin D supplementation groups and 2,389 patients in the control groups.
3.2 Study characteristics
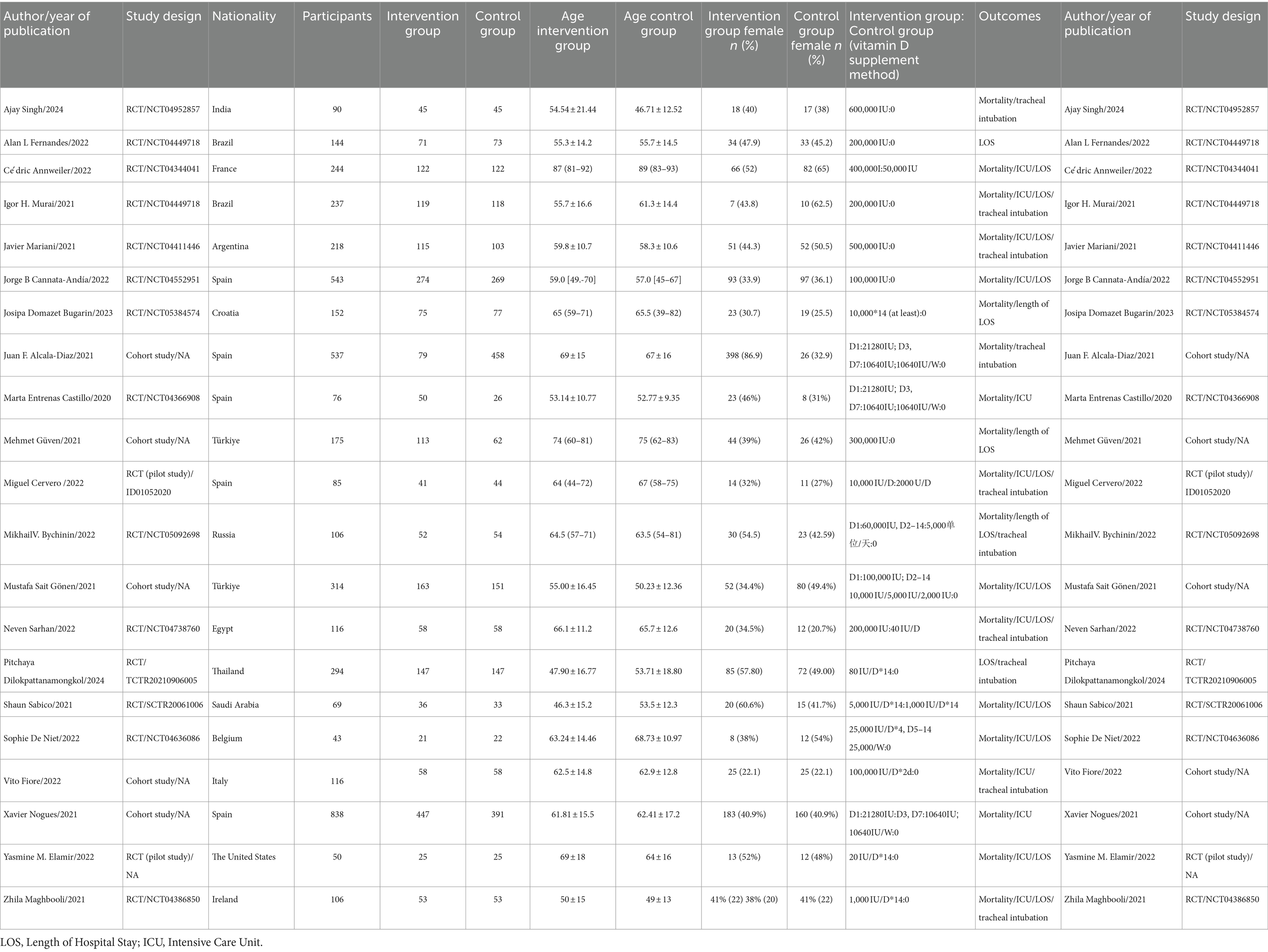
There were a total of 21 studies from Spain (n = 5), Turkey (n = 2), Brazil (n = 2), France (n = 1), Argentina (n = 1), Croatia (n = 1), Egypt (n = 1), Saudi Arabia (n = 1), Belgium (n = 1), Italy (n = 1), the United States (n = 1), Ireland (n = 1), Russia (n = 1), Thailand (n = 1), and India (n = 1). Among them, 16 were randomized controlled trials and five were cohort studies.
Of the 4,553 participants, 2,164 were in intervention groups and 2,389 were in control groups. The ages of the participants ranged from 31.1 to 93 years. Nineteen studies reported on participant mortality (14, 16–21, 23, 24, 31, 32, 34–36, 38–42), involving 4,115 participants. Thirteen studies reported on ICU admission rates (14, 17, 19, 21, 23, 31, 35, 36, 38–41) and included 2,811 participants. There were 15 studies involving 2,652 participants that assessed the length of hospital stay (14, 17–21, 23, 24, 32–35, 37, 39, 41). Ten studies reported on intubation rates (16, 20, 21, 31–33, 38, 39, 41, 42), with 1,995 participants. Among the 21 clinical trials, the intervention groups received varying daily doses of Vitamin D, ranging from 20 IU to 600,000 IU. In contrast, the control groups in two clinical trials received small doses of Vitamin D orally, relative to the intervention group. The remaining 19 trials used either a placebo or no intervention. In eight clinical trials, the intervention group members were administered Vitamin D as a single dose upon admission, while the remaining 13 trials involved continuous Vitamin D supplementation. The intervention group members of 13 clinical trials received a total Vitamin D dose of greater than or equal to 100,000 IU in the 14 days after hospitalization, while the patients in the remaining eight trials received a total dose of less than 100,000 IU. Table 1 offers further details of the study characteristics.
3.3 Risk of bias
3.3.1 Methods section
To assess the risk of bias in the included studies, we used the Cochrane Risk of Bias (RoB) assessment tool, which evaluates six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Disagreements during the assessment process were resolved through discussion, and if consensus could not be reached, arbitration was conducted by a third reviewer. We also performed sensitivity analyses to evaluate the robustness of the study results, including the exclusion of studies with high risk of bias and studies with extreme effect sizes or outliers. Meta-analyses were conducted using RevMan 5.3 software, generating an overall risk of bias graph.
3.3.2 Results section
Among the 21 clinical trials included, random sequence generation in 15 randomized controlled trials (RCTs) was deemed low risk of bias, while three cohort studies were classified as high risk, and three were uncertain. Regarding allocation concealment, 16 studies were considered low risk, one did not report allocation methods and was thus deemed uncertain, and four were classified as high risk. Four RCTs did not employ blinding, four did not mention blinding, and none of the cohort or observational studies applied blinding. The risk of bias in outcome blinding assessment was generally low in the RCTs; specifically, 11 studies conducted double-blind trials, 5 did not implement double-blinding, and 5 did not clearly specify their blinding procedures. In cohort and observational studies, outcome blinding was not explicitly mentioned. A detailed summary of these findings is provided in Figures 2A,B.
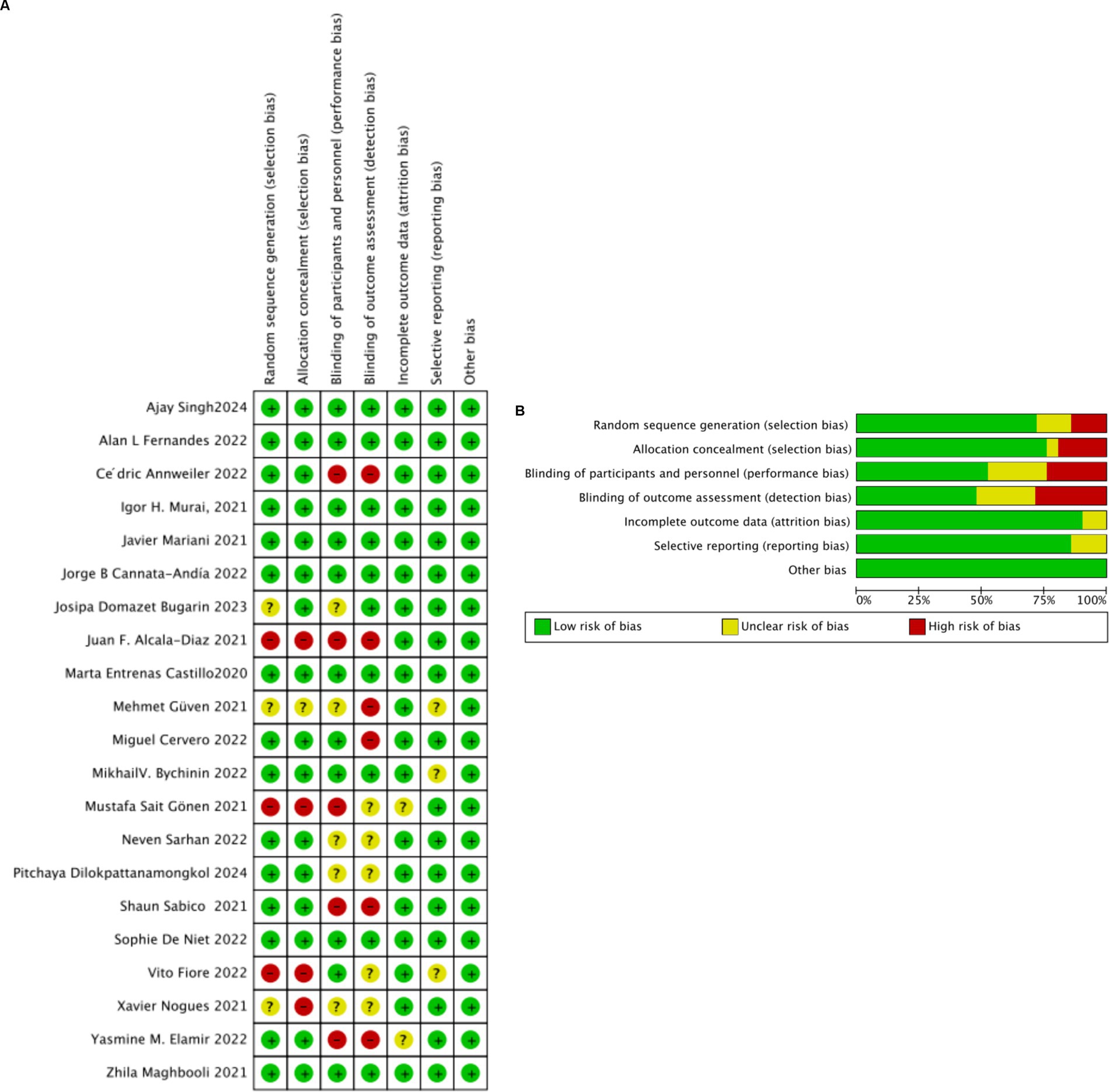
Figure 2. (A) Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. (B) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
3.3.3 Sensitivity analysis
We performed sensitivity analyses to evaluate the robustness of our findings by excluding studies with a high risk of bias or outliers. The results indicated that the main outcomes, such as mortality and ICU admission rates, remained consistent even after excluding these high-risk or extreme studies. This suggests that our findings are robust and not influenced by individual studies with a high risk of bias or extreme values.
3.4 Meta-analyses
3.4.1 Effect of oral vitamin D on the mortality rate of COVID-19-infected individuals
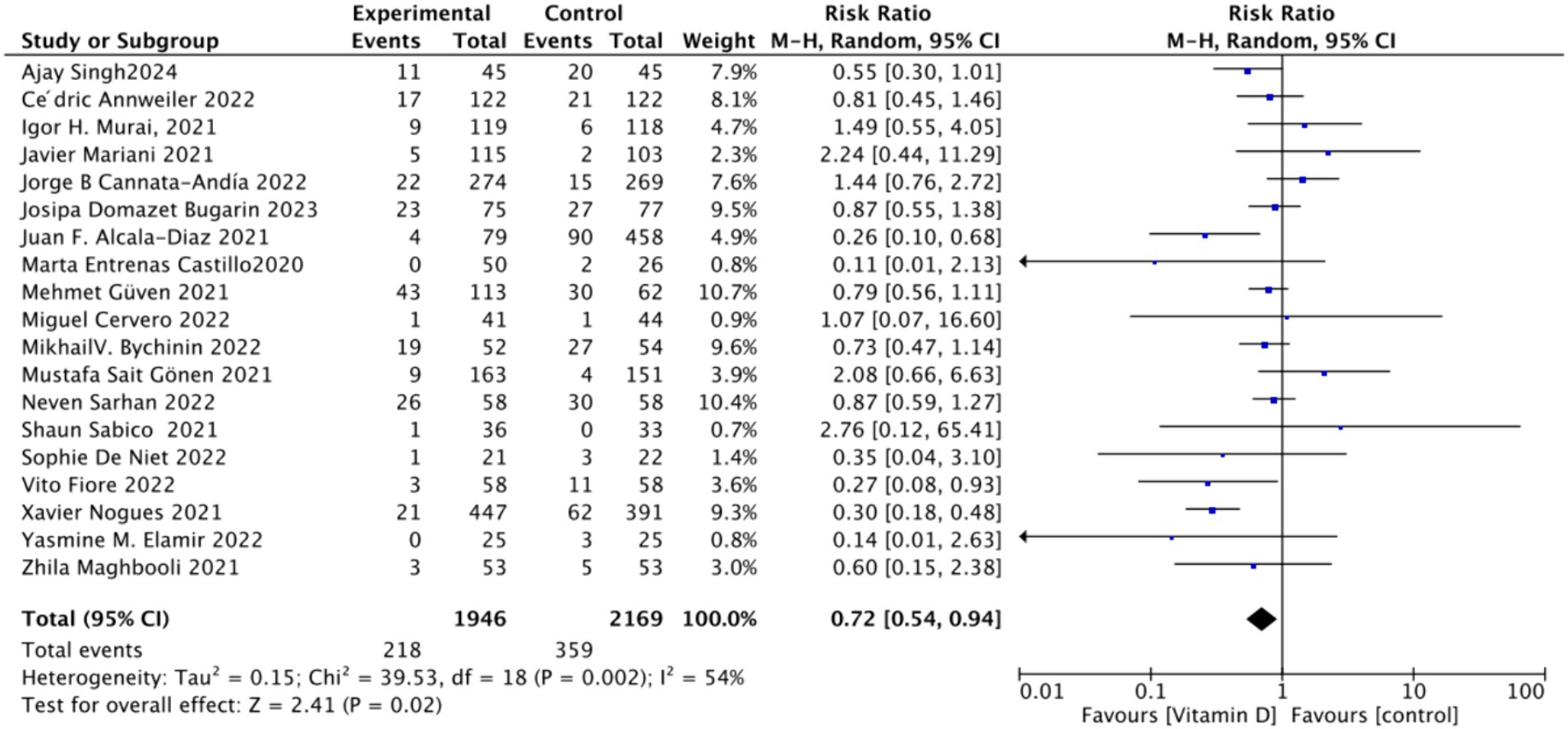
A. To analyze the mortality rate, we included 19 studies with a total of 4,115 participants. We first conducted a heterogeneity test, which revealed an I2 value greater than 50% (I2 = 54%, p = 0.02). As a result, we employed a random-effects model for statistical analysis. Data analysis showed a total relative risk (RR) of 0.72 (95% CI: 0.54–0.94), with a significance level of p = 0.02 (Figure 3).
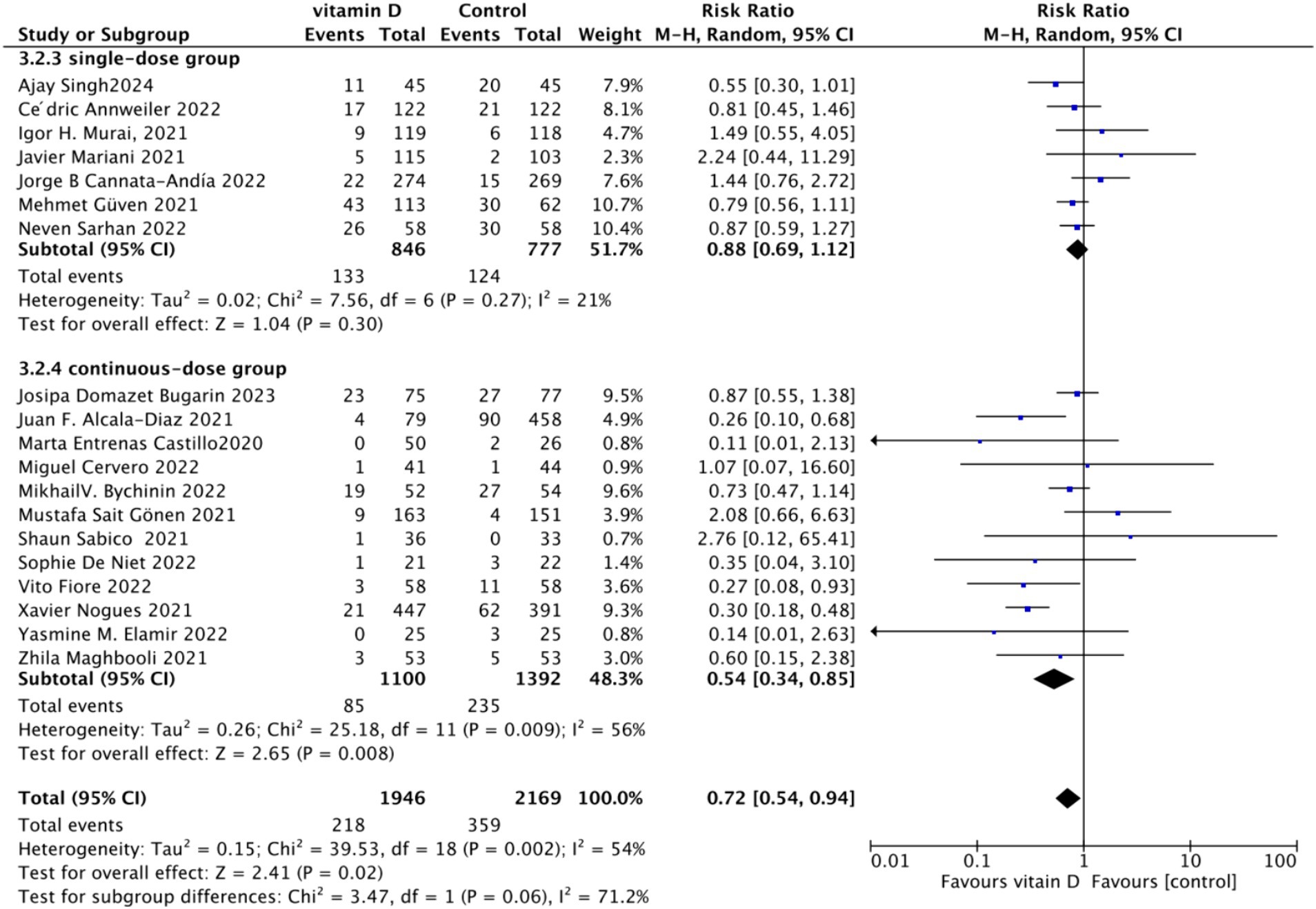
B. Subgrouped by administration method: For the subgroup analysis based on the mortality rate, we categorized the intervention groups into two subgroups according to the Vitamin D intake method: single dose or continuous dose. Studies administering Vitamin D only once upon admission were classified as the single dose group (dose frequency = 1), while those administering multiple doses after admission were classified as the continuous dose group (dose frequency ≧ 2). Seven studies employed a single-dose regimen, while 12 studies used a continuous-dose regimen. The combined relative risk (RR) for the single-dose subgroup of the intervention group was 0.88 (95% CI: 0.69–1.12), with an I2 value of 21% and p = 0.3, indicating no statistically significant reduction in mortality. Conversely, the RR for the continuous-dose subgroup was 0.53 (95% CI: 0.34–0.83), with an I2 value of 55% and p = 0.006, showing a statistically significant reduction in mortality (Figure 4).
C. Subgrouped by dosage: For the second subgroup analysis based on mortality rate, we categorized the intervention groups according to the total Vitamin D intake. This was calculated according to the total dosage during the first 14 days of hospitalization. There were two groups: ≥100,000 International Units (IU and <100,000 IU). Of the selected studies, 12 administered total doses of ≥100,000 IU of Vitamin D in the intervention group, while seven administered <100,000 IU. The combined RR value for the ≥100,000 IU intake group was 0.85 (95% CI: 0.71–1.02), with an I2 value of 1% and p = 0.07, indicating no statistically significant reduction in mortality. In contrast, the RR for the <100,000 IU intake group was 0.30 (95% CI: 0.21–0.44), with an I2 value of 0% and p < 0.0001, showing a statistically significant reduction in mortality (Figure 5).
D. Subgrouped by serum Vitamin D concentrations upon admission: For the third subgroup analysis based on mortality rate, we classified the studies according to the serum Vitamin D concentration restrictions applied to the included patients. The groups were divided into those with Vitamin D deficiency (serum 25 (OH)D (25-Hydroxyvitamin D) < 30 ng/mL) and those with no restrictions on serum Vitamin D concentrations. Of the selected studies, 9 limited inclusion to patients with serum 25 (OH) D < 30 ng/mL, while the other 10 studies had no restrictions on serum 25 (OH) D levels. The combined risk ratio (RR) for the group with serum 25 (OH) D < 30 ng/mL was 0.73 (95% CI: 0.59–0.89), with an I2 value of 0% and p = 0.002, indicating a statistically significant reduction in mortality. In contrast, the RR for the group with no restrictions on serum 25 (OH) D concentrations was 0.73 (95% CI: 0.46–1.15), with an I2 value of 73% and p = 0.18, suggesting no statistically significant reduction in mortality (Figure 6).
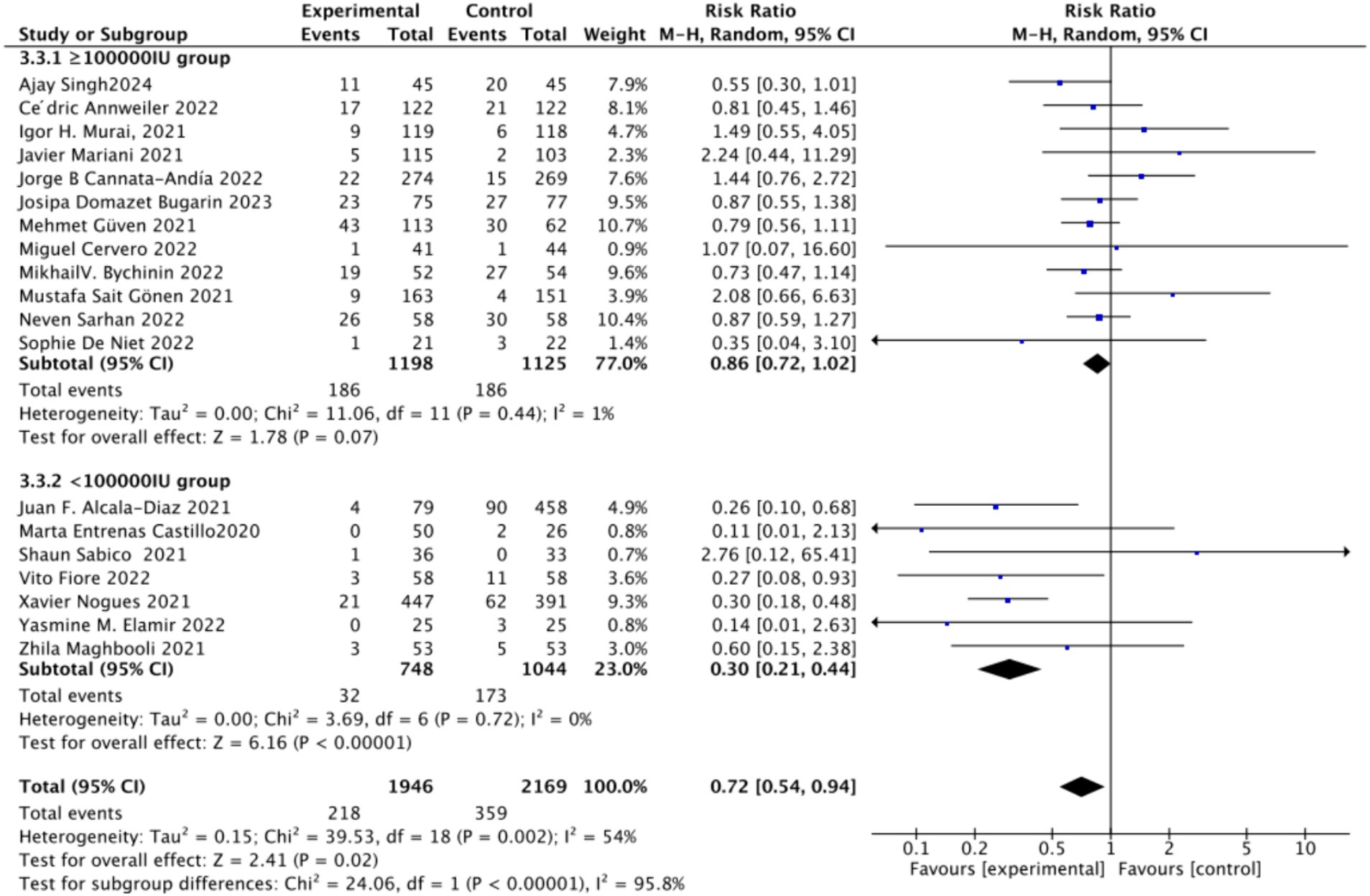
Figure 5. Forest plot of mortality rate by total Vitamin D dosage intake within the first 14 days of hospitalization.
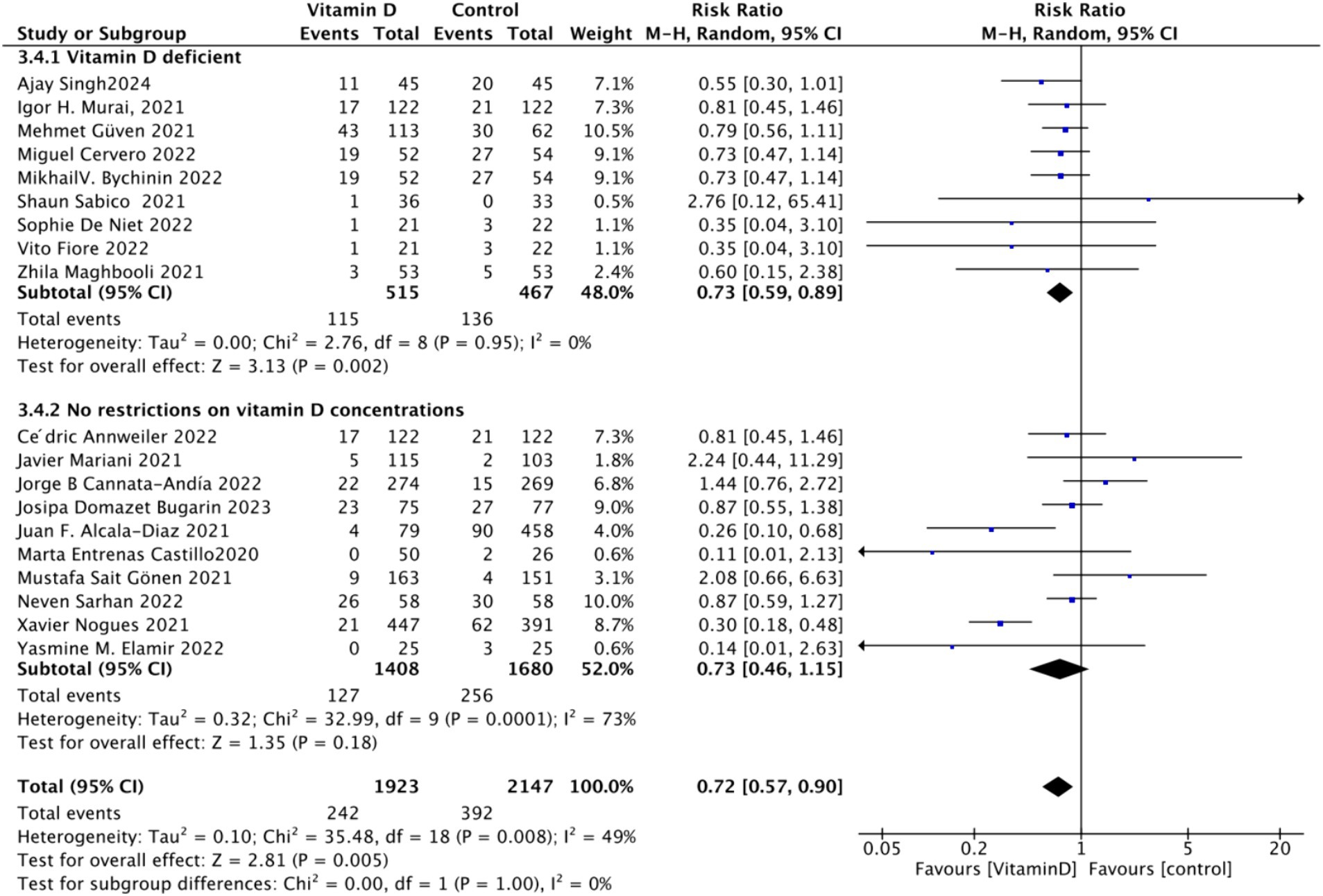
Figure 6. Forest plot of mortality rate by baseline serum Vitamin D concentration restrictions among study populations.
3.4.2 Effect of oral vitamin D dosage on the risk of ICU admission
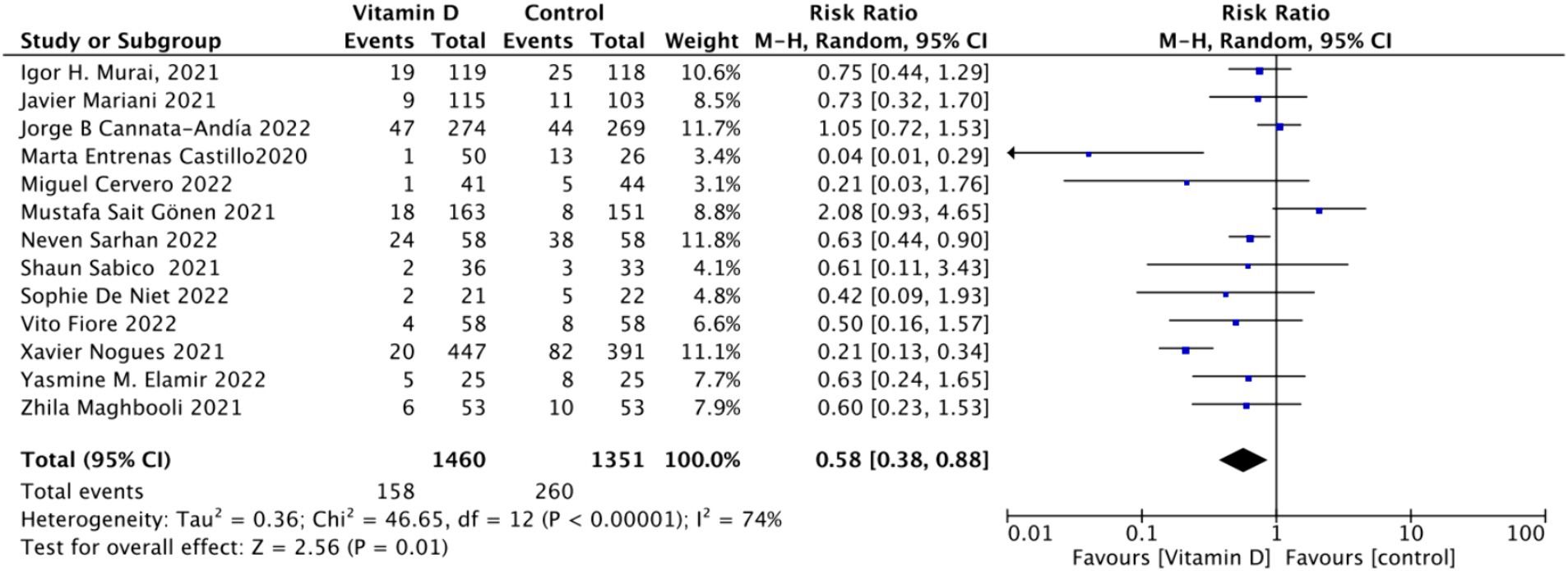
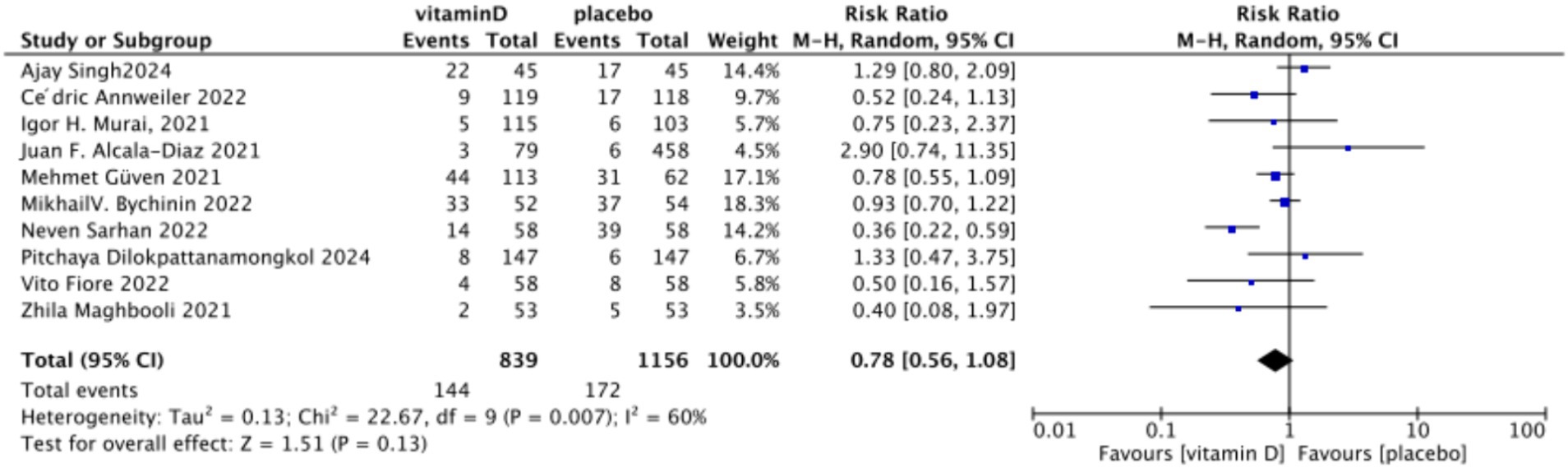
A. When assessing ICU admission rate as an outcome, a total of 13 studies involving 2,811 participants were included. The pooled relative risk (RR) was 0.58 (95% CI: 0.38–0.88), with significant heterogeneity (I2 = 74%, p = 0.01) (Figure 7).
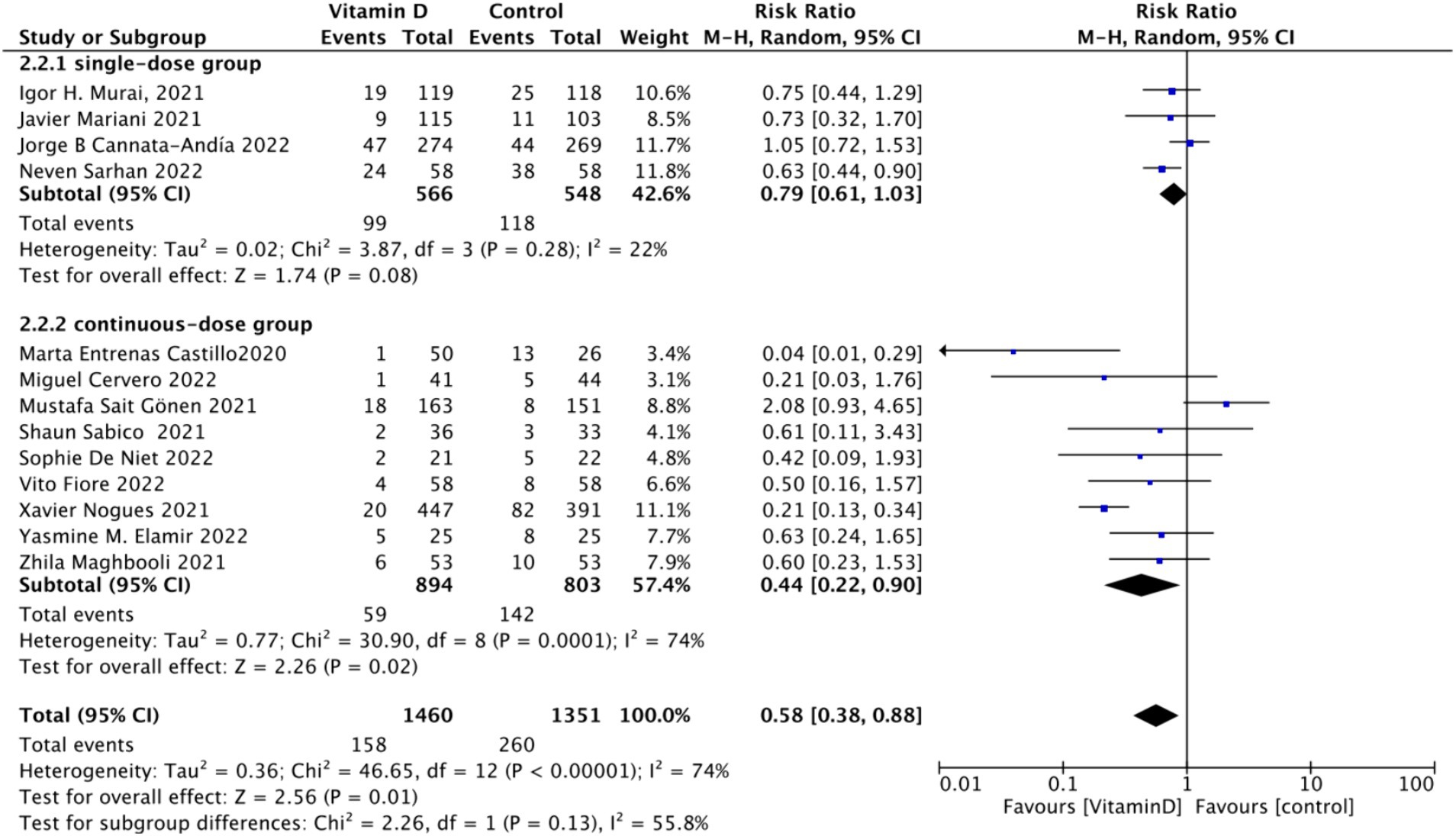
B. Subgrouped by administration method: Of the included studies, four applied single-dose administration, while nine used continuous administration. The combined RR value for the single-dose Vitamin D subgroup was 0.79 (95% CI: 0.61–1.03), with low heterogeneity (I2 = 22%, p = 0.08), indicating no statistically significant reduction in ICU admission rates. In contrast, the combined RR for the continuous administration subgroup was 0.44 (95% CI: 0.22–0.90), with substantial heterogeneity (I2 = 74%, p = 0.02), showing a statistically significant reduction in ICU admission rates (Figure 8).
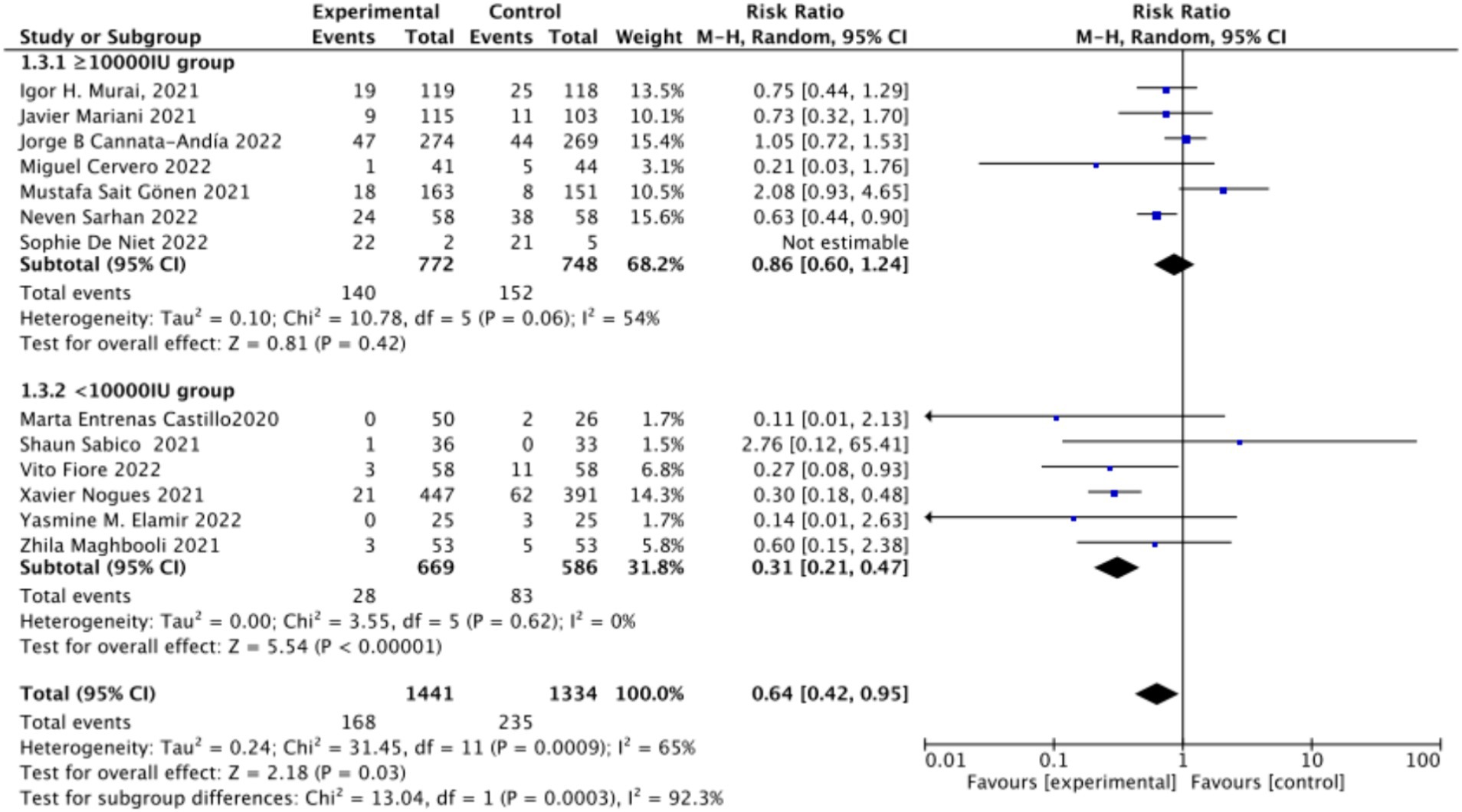
C. Subgrouped by dosage: The studies were further subdivided according to the Vitamin D dosage. Seven studies utilized Vitamin D dosages of ≥100,000 IU, while six offered dosages of <100,000 IU. The combined RR value for the ≥100,000 IU subgroup over 14 days was 0.86 (95% CI: 0.6–1.24), with moderate heterogeneity (I2 = 54%, p = 0.42), indicating no statistically significant reduction in ICU admission rates. However, the combined RR for the <100,000 IU subgroup was 0.31 (95% CI: 0.21–0.47), exhibiting low heterogeneity (I2 = 0%, p = 0.001), showing a statistically significant reduction in ICU admission rates (Figure 9).
D. Subgrouped by serum Vitamin D concentrations upon admission: Among the selected studies, 6 restricted inclusion to patients with serum 25 (OH) D levels <30 ng/mL, while the remaining 7 studies imposed no restrictions on serum 25 (OH) D levels. The pooled risk ratio (RR) for the group with serum 25 (OH) D < 30 ng/mL was 0.63 (95% CI: 0.42–0.93), I2 = 0%, and p = 0.02, indicating a statistically significant reduction in ICU admission. Conversely, for the group with no restrictions on serum 25 (OH) D concentrations, the RR was 0.59 (95% CI: 0.32–1.11), (I2 = 86%) and p = 0.1, indicating no statistically significant reduction in ICU admission (Figure 10).

Figure 10. Forest plot of ICU admission by baseline serum Vitamin D concentration restrictions among study populations.
3.4.3 Effect of oral vitamin D on hospitalization duration
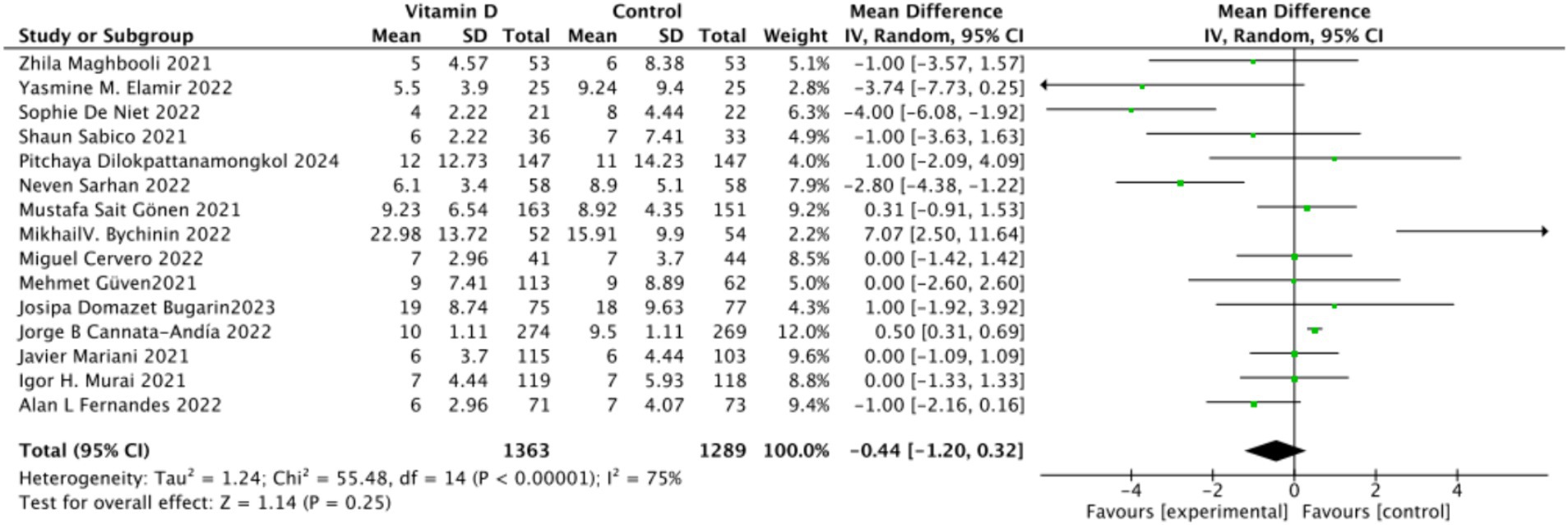
A. There were 15 studies involving 2,652 participants that assessed hospitalization duration as an outcome. The pooled results showed no significant difference between the Vitamin D group and the control group, with a standardized mean difference (MD) of −1 (95% CI: −2.16 to 0.16; p = 0.13) (Figure 11).
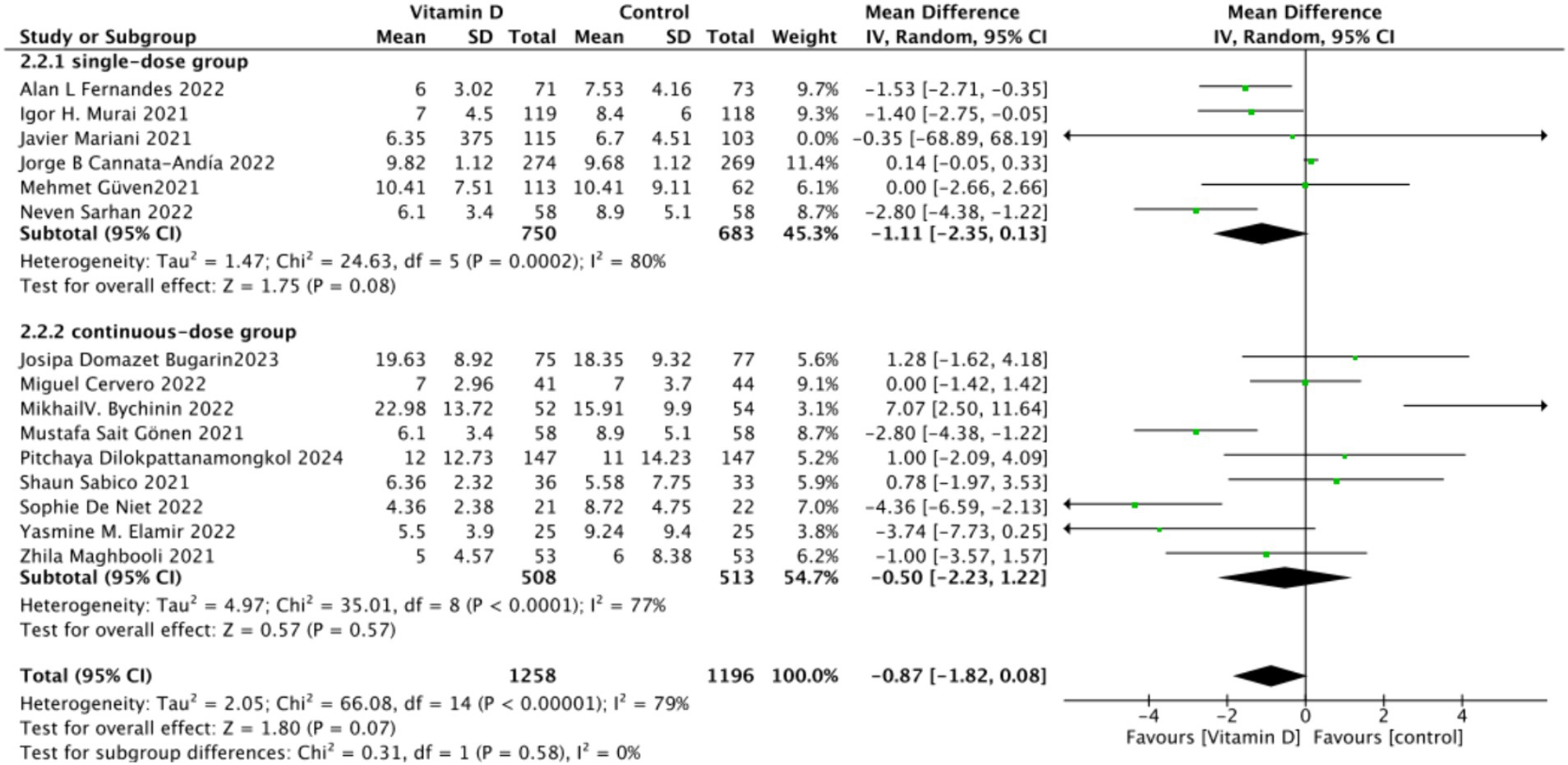
B. Subgrouped by administration method: Six studies administered Vitamin D in single doses, while nine studies offered them continuously. There was a negligible difference between the single-dose subgroup and the control group, with an MD of −1.11 (95% CI: −2.35 to 0.13; p = 0.08). Similarly, there was no significant difference between the subgroup receiving continuous Vitamin D administration and the control group, with an MD of −0.50 (95% CI: −2.23 to 1.22; p = 0.57) (Figure 12).
C. Subgrouped by dosage: Ten studies administered Vitamin D dosages of ≥100,000 IU, while three studies gave <100,000 IU. There was no significant difference between the subgroup receiving dosages of ≥100,000 IU and the control group, with an MD of −0.91 (95% CI: −1.83 to 0.01; p = 0.05). Similarly, there was an insignificant difference between the subgroup receiving <100,000 IU and the control group, with an MD of −0.97 (95% CI, −3.24 to 1.29; p = 0.4) (Figure 13).
D. Subgrouped by serum Vitamin D concentrations upon admission: Among the selected studies, 8 restricted inclusion to patients with serum 25(OH) D levels <30 ng/mL, while the remaining 7 studies imposed no restrictions on serum 25(OH)D levels, to evaluate the impact on the length of hospital stay. The pooled mean difference for the group with serum 25(OH)D < 30 ng/mL was −0.62 (95% CI: −2.17 to 0.92), with an I2 of 72% and p = 0.43. For the group with no restrictions on serum 25(OH)D concentrations, the mean difference was −0.25 (95% CI: −1.12 to 0.62), with an I2 of 75% and p = 0.57 (Figure 14).
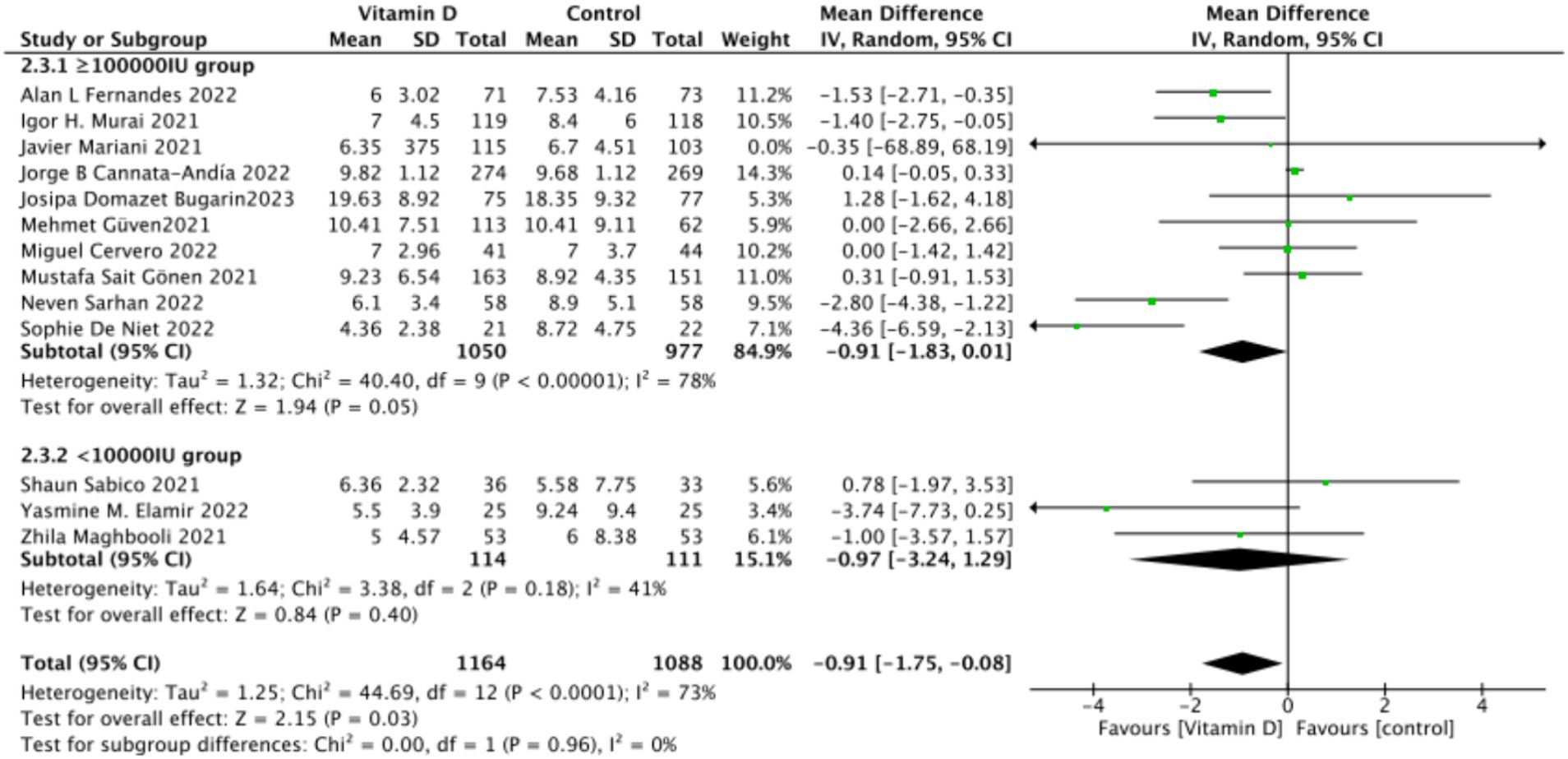
Figure 13. Forest plot of hospital stay duration subgrouped by total Vitamin D dosage administered within 14 days of admission.
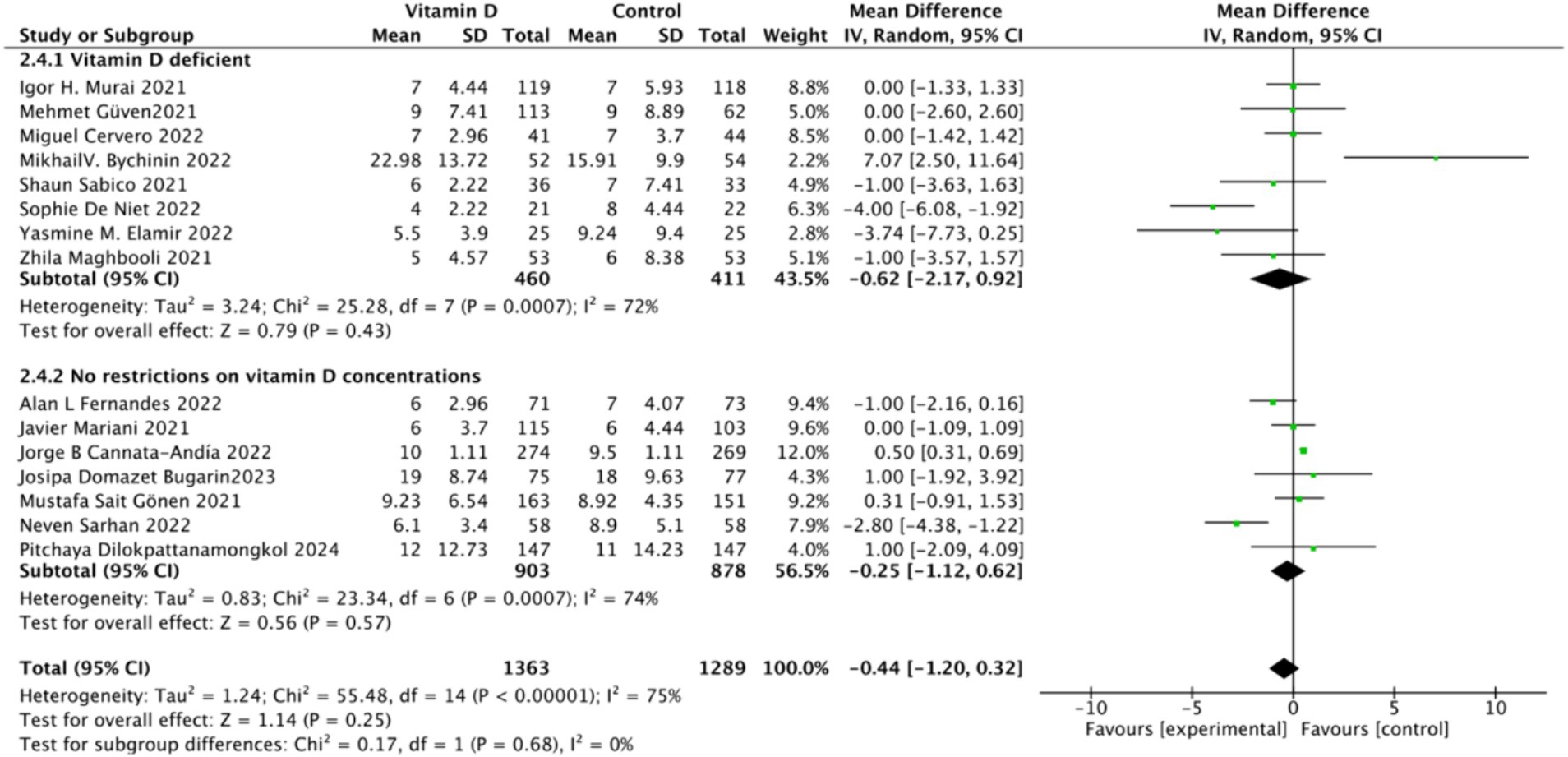
Figure 14. Forest plot of hospital stay duration subgrouped by baseline serum Vitamin D concentration.
3.4.4 Impact of oral vitamin D on endotracheal intubation rate
A. In the analysis of endotracheal intubation, 10 studies involving 1,995 participants were included. The results indicated no significant difference between the Vitamin D group and the control group, with an RR value of 0.78 (95% CI: 0.56, 1.08; p = 0.13) (Figure 15).
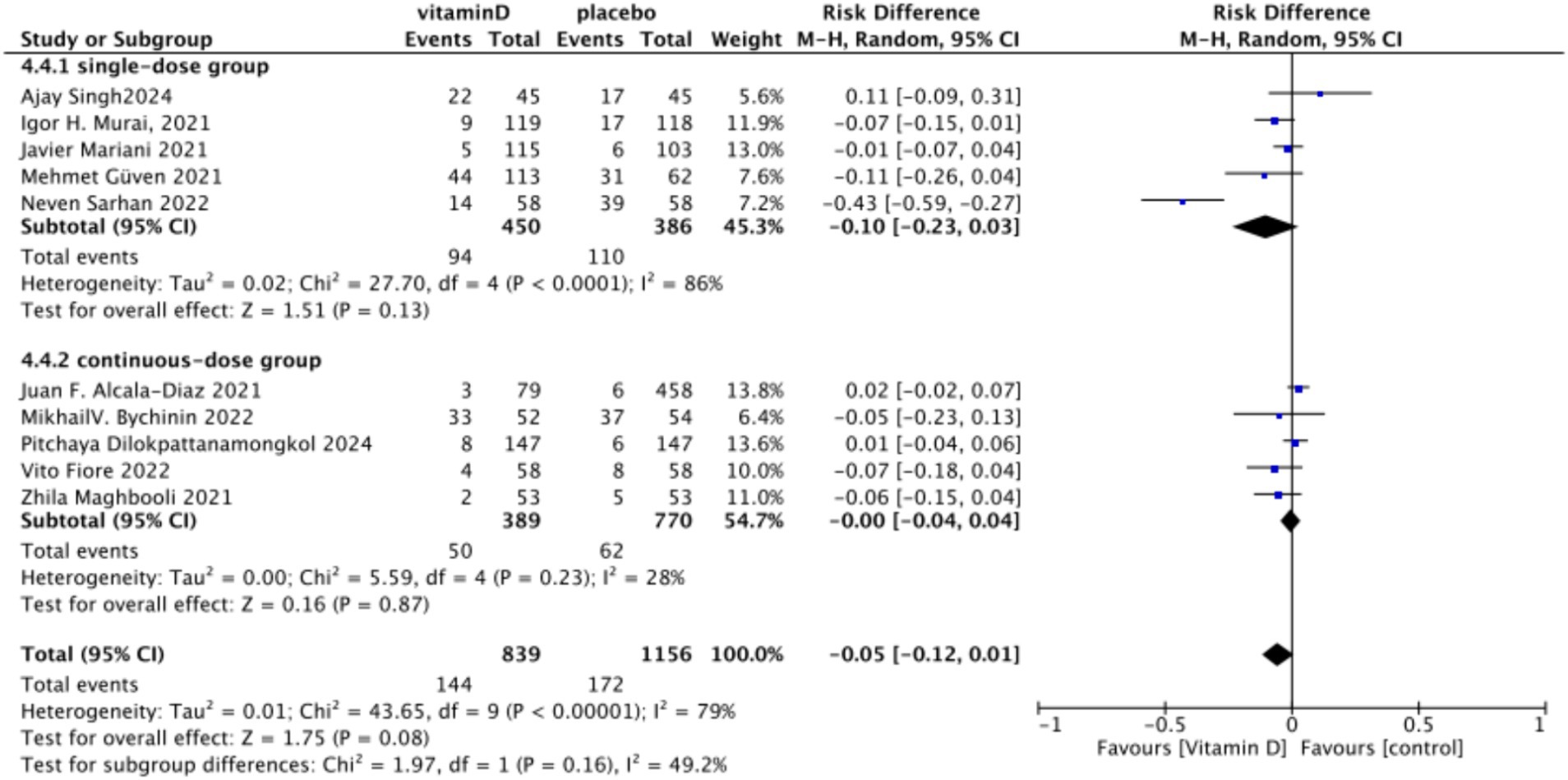
B. Subgrouped by administration method: Five studies involved one-time doses and five studies utilized continuous dosing. There was a negligible difference between the intervention group with one-time Vitamin D dosage and the control group. Specifically, the RR value was −0.10 (95% CI, −0.23, 0.03; p = 0.13). Similarly, no significant difference was observed between the intervention group with continuous Vitamin D dosage and the control group, with an RR value of −0.00 (95% CI: −0.04 to 0.04; p = 0.87) (Figure 16).
C. Subgrouped by dosage: Six studies included intervention groups with Vitamin D dosages ≥100,000 IU, while four studies involved groups with dosages <100,000 IU. There were no significant differences between the intervention group with dosages of ≥100,000 IU and the control group. The RR value was 0.59 (95% CI: 0.32, 1.09; p = 0.09). Equally, no noticeable differences were observed between the intervention group with Vitamin D dosages of <100,000 IU and the control group, with an RR value of 0.96 (95% CI: 0.39–2.35; p = 0.93) (Figure 17).
D. Subgrouped by serum Vitamin D concentrations upon admission: Among the selected studies, 6 restricted inclusion to patients with serum 25(OH)D levels <30 ng/mL, while the remaining 4 studies imposed no restrictions on serum 25(OH)D levels, to evaluate the impact on the of intubation. The pooled risk ratio (RR) for the group with serum 25(OH)D < 30 ng/mL was 0.92 (95% CI: 0.61 to 1.37), with an I2 of 36% and p = 0.67. For the group with no restrictions on serum 25(OH)D concentrations, the RR was 0.78 (95% CI: 0.34 to 1.80), with an I2 of 74% and p = 0.57 (Figure 18).
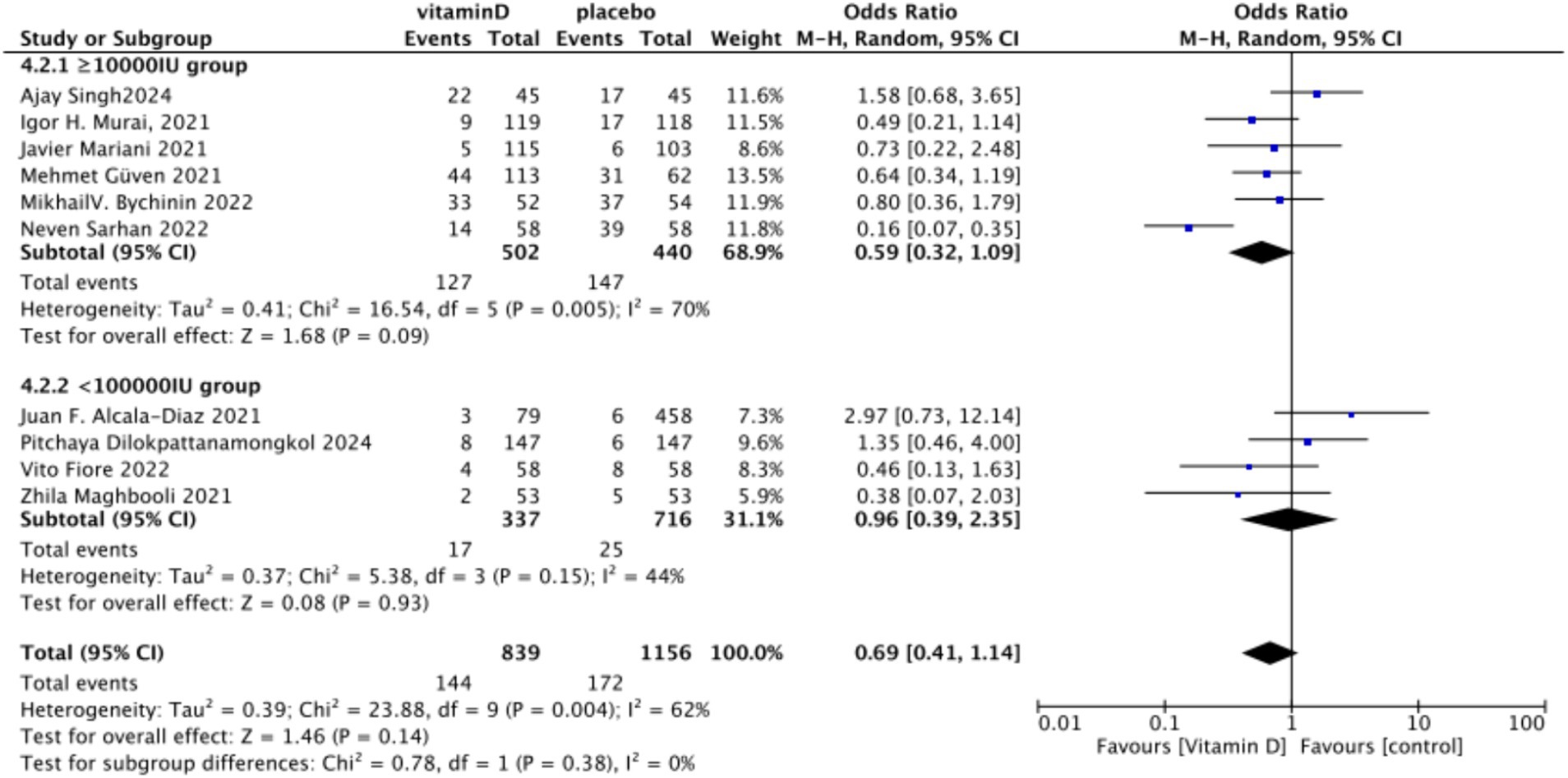
Figure 17. Forest plot of tracheal intubation rate by subgroup according to total Vitamin D dose administered within 14 days of admission.
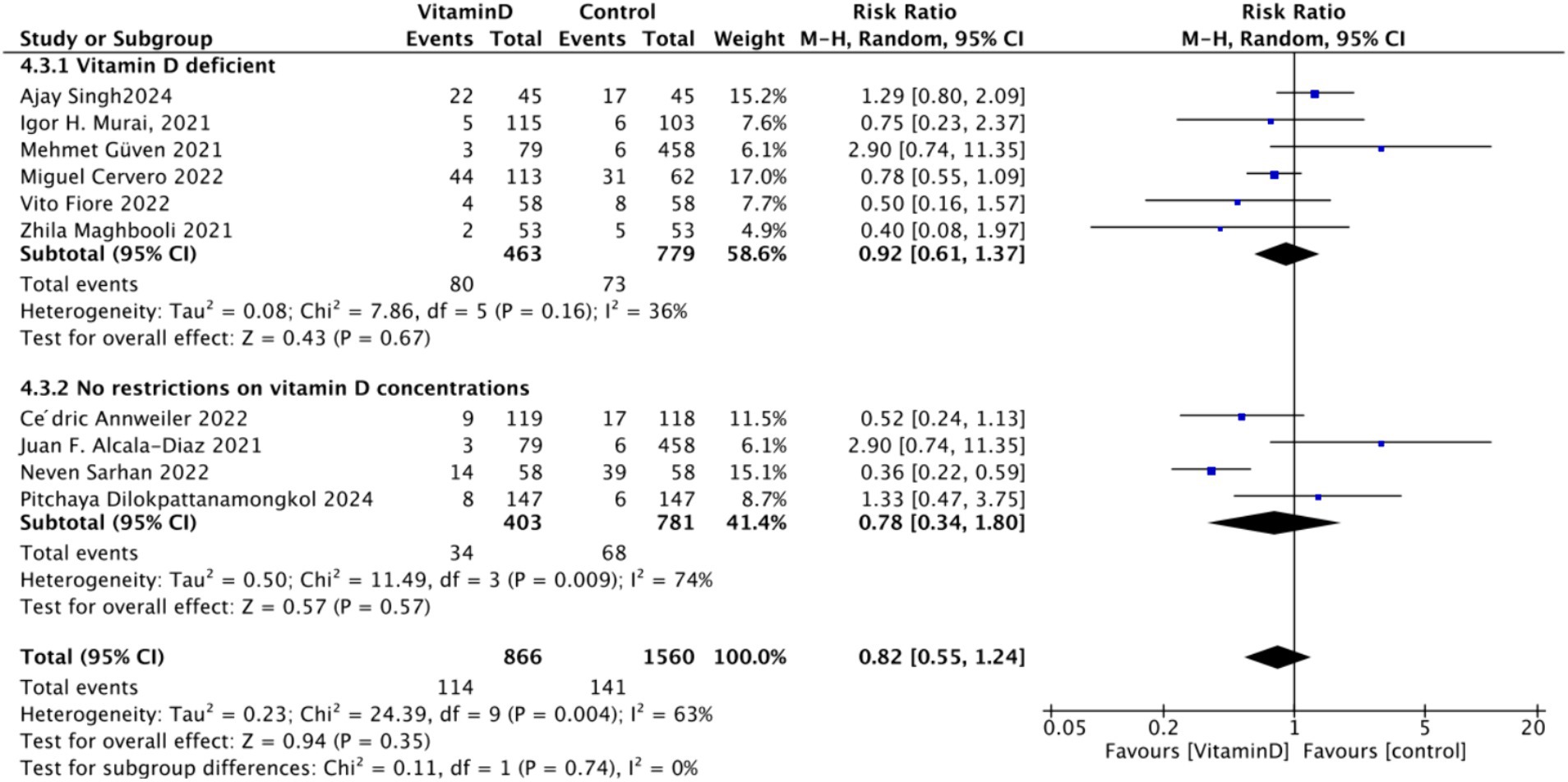
Figure 18. Forest plot of tracheal intubation rate by subgroup according to baseline serum Vitamin D concentrations.
3.5 Administration method
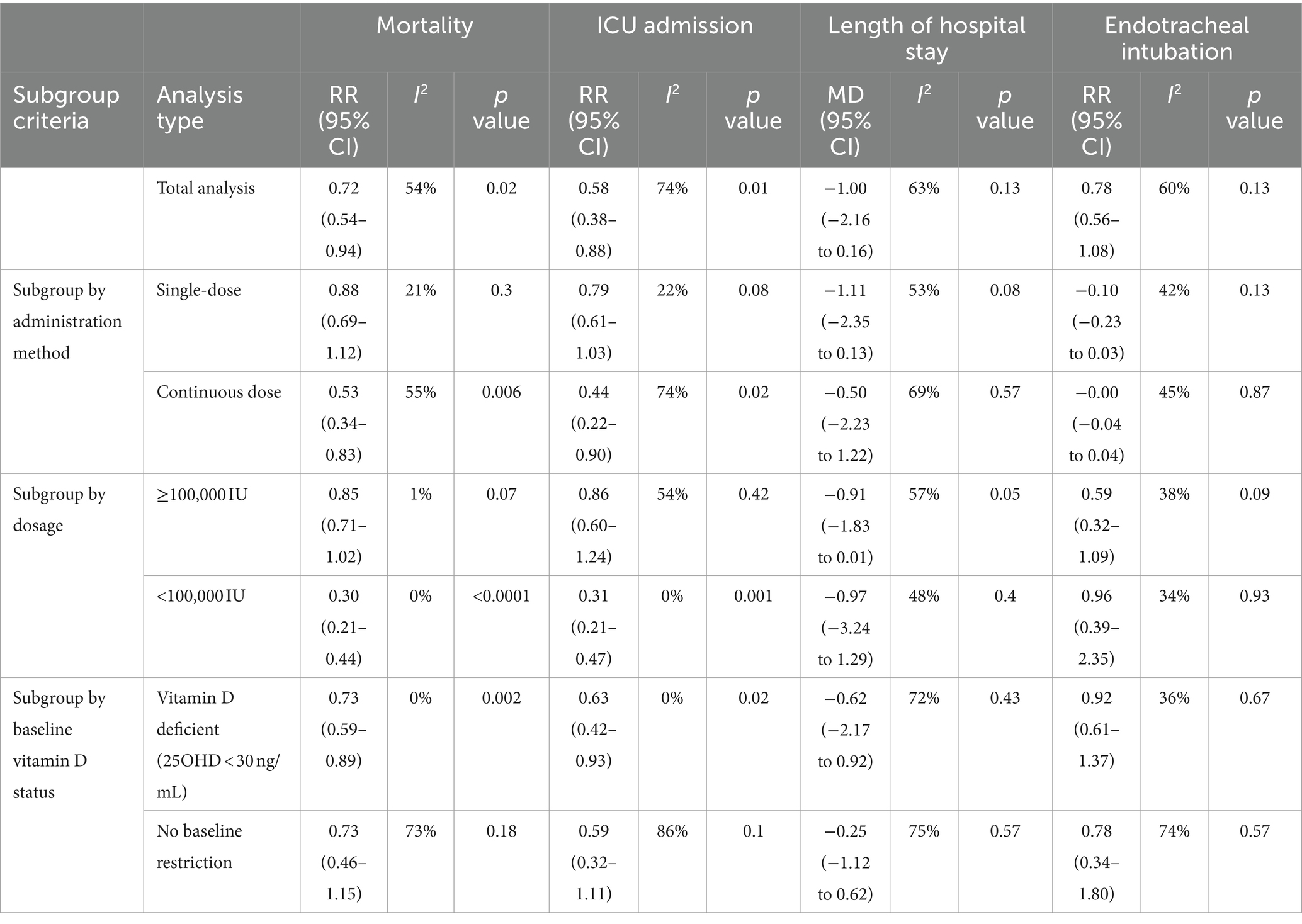
Continuous, multiple-dose administration resulted in lower heterogeneity (I2 = 55% for mortality, I2 = 74% for ICU admissions) and significant reductions in both mortality (p = 0.006) and ICU admissions (p = 0.02). Single-dose administration, which showed higher heterogeneity and no significant improvements, underscores that continuous dosing provides more consistent and effective results.
Total Dosage Over 14 Days: Lower dosages (<100,000 IU) led to reduced heterogeneity (I2 = 0% for both mortality and ICU admissions) and significant outcome improvements (p < 0.0001 for mortality, p = 0.001 for ICU admissions). Higher dosages (≥100,000 IU) showed moderate heterogeneity and did not achieve similar benefits, indicating that moderate dosing not only improves outcomes but also provides more consistent results.
Baseline Vitamin D Status: Patients with Vitamin D deficiency (25OHD < 30 ng/mL) showed low heterogeneity and significant benefits from supplementation, including reduced mortality (I2 = 0%, p = 0.002) and ICU admission rates (I2 = 0%, p = 0.02). In contrast, high heterogeneity in the no-restriction group (I2 = 73% for mortality, I2 = 86% for ICU admission) with no significant benefits suggests that baseline deficiency is crucial for achieving effective outcomes (Table 2).
3.6 Bias analysis
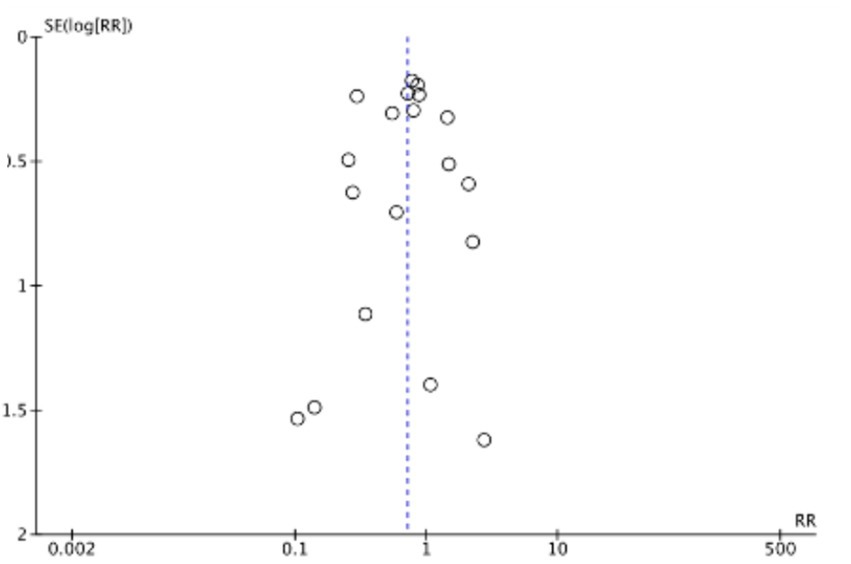
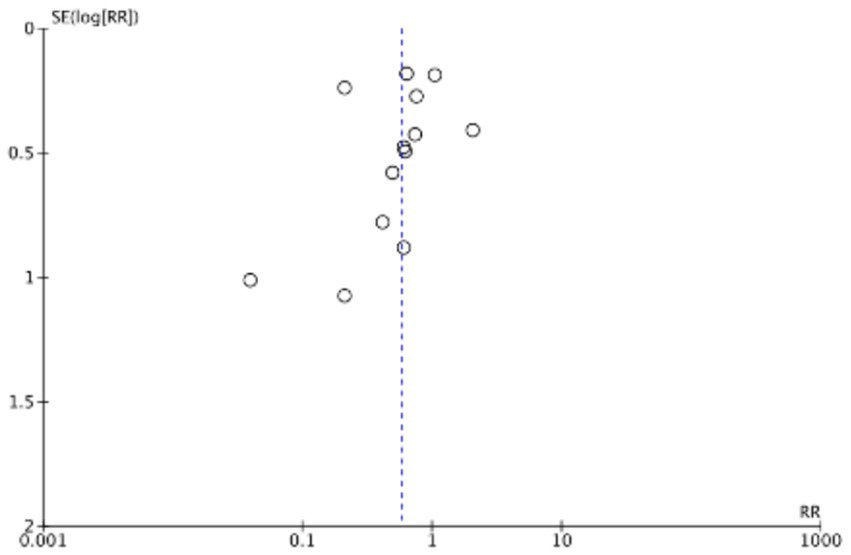
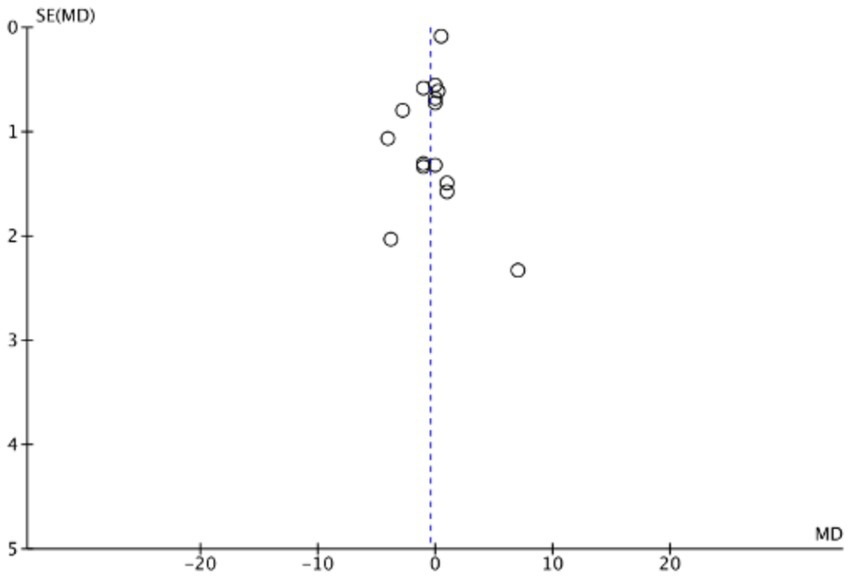
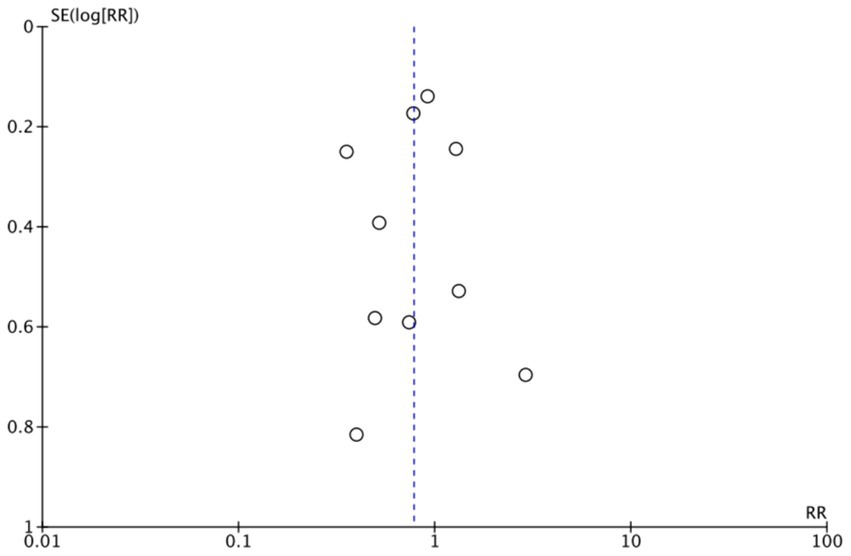
The funnel plots (Figures 19–22) are approximately symmetrical on both sides, indicating the absence of meaningful publication bias. Subsequently, we conducted a sensitivity analysis, systematically assessing the impact of removing individual studies. We established that no single study exerted substantial influence on the overall results, suggesting that our findings were robust.
4 Discussion
In this systematic review and meta-analysis, we investigated the impact of oral Vitamin D supplementation on the prognosis of COVID-19 patients across 21 studies involving 4,553 individuals. Our findings suggest that Vitamin D administration following COVID-19 infection leads to improved mortality rates and reduced ICU admission rates. However, we did not observe any significant effects on hospital stay duration or intubation rates.
To achieve the optimal therapeutic effect of Vitamin D supplementation, we conducted various subgroup analyses. Patients were classified based on three main criteria: the mode of Vitamin D administration upon hospital admission (single-dose versus multiple-dose), the total Vitamin D dosage over 14 days (≥100,000 IU versus <100,000 IU), and baseline Vitamin D levels (serum 25(OH)D < 30 ng/mL versus no restriction). Our analysis revealed that continuous, multiple-dose administration and total dosages of <100,000 IU over 14 days were strongly associated with reduced mortality and ICU admission rates. In contrast, single-dose administration and total dosages ≥100,000 IU did not show noticeable improvements in outcomes. Furthermore, patients with baseline Vitamin D deficiency (25OHD < 30 ng/mL) experienced significant reductions in mortality and ICU admission rates following supplementation, while those without baseline restrictions did not show the same level of benefit.
By implementing strategic subgroup analyses, we effectively reduced heterogeneity and achieved more consistent results. For example, grouping patients by total Vitamin D dosage over 14 days and baseline Vitamin D levels significantly lowered heterogeneity. The <100,000 IU total dose subgroup and the Vitamin D deficiency subgroup (25OHD < 30 ng/mL) frequently showed heterogeneity values of zero in mortality and ICU admission outcomes (Table 2). These findings suggest that lower, continuous doses of Vitamin D are more effective than higher, single doses and that supplementation in Vitamin D-deficient populations leads to greater improvements in clinical prognosis. By highlighting the importance of dosing strategies and baseline Vitamin D status, our study provides a potential explanation for the conflicting results observed in previous research on Vitamin D supplementation and COVID-19 outcomes. The inconsistencies in earlier studies may be attributed to differences in patient selection, dosing regimens, and a lack of consideration for baseline Vitamin D status.
Since the outbreak of the novel coronavirus infection in 2019, numerous studies have explored the relationship between Vitamin D supplementation and the prognosis for COVID-19 infection. However, results from clinical trials vary and meta-analyses on this topic also exhibit discrepancies. For instance, the majority of meta-analyses conclude that oral Vitamin D supplementation has a negligible impact on the mortality rate of COVID-19 patients (22, 43–51). Nevertheless, a few reports suggest that supplementation reduces COVID-19 patient mortality rates (52, 53). Similarly, most meta-analyses state that Vitamin D supplementation significantly lowers the ICU admission rate of COVID-19 patients (43, 46, 47, 49–51, 53), although some studies contradict these findings (22, 45). These discrepancies may be due to the Vitamin D supplementation dosage and method, necessitating further clinical trials and meta-analyses for deeper investigation. Our study evaluated the mortality rate, ICU admission rate, length of hospital stay, and intubation rate of hospitalized COVID-19 patients. The positive feature of this study is that we conducted subgroup analyses based on the mode of administration and 14-day total Vitamin D intake, which appreciably reduced the heterogeneity in some subgroups. Additionally, our study yielded new findings regarding total intake, partially clarifying the discrepancies encountered in previous meta-analyses.
Our conclusions regarding the impact of administration methods on the clinical outcomes of COVID-19 patients suggest that continuous administration of Vitamin D is superior to one-time bolus intake. The relationship between Vitamin D supplementation methods and respiratory tract infections has been studied extensively in recent years. Numerous clinical trials have consistently shown that continuous low-dose Vitamin D supplementation is more effective than intermittent high-dose administration. In their respective meta-analyses of numerous randomized controlled trials, Martineau (54) and Jolliffe (6) both concluded that continuous low-dose maintenance Vitamin D supplementation produces a considerably greater protective effect against the risk of acute respiratory tract infections compared to high-dose intermittent dosing. Considering that COVID-19 is a respiratory infection, during the early stages of the COVID-19 pandemic, Griffin (55) recommended continuous administration of Vitamin D for COVID-19 patients based on the relationship between Vitamin D supplementation and respiratory tract infections. Elaborating on this, Feiner Solís et al. (56) conducted a systematic review, summarizing 11 relevant clinical trials. Six of the studies involved continuous Vitamin D administration and five studies involved a single bolus dose. His results revealed that continuous Vitamin D supplementation was associated with better clinical outcomes in patients with COVID-19, whereas a single bolus dose did not improve any clinical outcomes. Our study further corroborates these findings. From the perspective of Vitamin D metabolism, a plausible explanation is that its activation and metabolism are regulated by enzymes. Following a large bolus dose of Vitamin D, the enzyme 24-hydroxylase, which inactivates Vitamin D, may remain active for several weeks as a feedback response (57). This sustained elevation in 24-hydroxylase activity can paradoxically result in intracellular depletion of active Vitamin D, known as the rebound effect, particularly affecting immune cells. In contrast, daily low-dose supplementation maintains consistent Vitamin D activity by preventing the significant upregulation of 24-hydroxylase (58).
To date, there have been no clinical trials or meta-analyses that specifically analyze the effects of total intake within a certain timeframe on the prognosis of COVID-19 patients. In the context of respiratory tract infections and Vitamin D supplementation, the RCT study by Wall-Gremstrup et al. (59) proposed that the probability of respiratory tract infections is significantly higher in the high-dose Vitamin D group (supplemented with 300,000 IU on the first day followed by 1,500 IU/day for the next 150 days) than the non-supplemented group. This study supports the notion that high-dose supplementation does not enhance immunity in infected patients and may even impair innate immunity. Vieth (60) provided a possible explanation from a pharmacological mechanism perspective. They suggested that high-dose Vitamin D3 intake leads to an imbalance in Vitamin D regulatory enzymes including CYP27B1 and CYP24A1 (Cytochrome P450 family 24 subfamily B member 1 and cytochrome P450 family 24 subfamily A member 1), resulting in significant fluctuations in serum 25(OH)D3 concentrations. This results in a fall in the levels of active Vitamin D (1,25(OH)2D3), which assists the immune system in combating respiratory tract infections. Therefore, high doses of Vitamin D may impair immune function. Another possible cause is fibroblast growth factor23 (FGF23), which is increased by high doses of oral Vitamin D but not by sustained low doses of Vitamin D of 2,000 IU or less per day. High concentration of FGF23 in turn significantly inhibited the 1α-hydroxylation of 25(OH)D, resulting in reduced Vitamin D intracellular activation of 1,25(OH)2D, thereby attenuating the immune-enhancing effect of Vitamin D (61). Concerning supplementation dosage and the prognosis of COVID-19 patients, Tentolouris et al. (47) conducted a preliminary analysis in their meta-analysis. They performed a subgroup analysis on single high-dose and low-dose Vitamin D supplementation, concluding that low-dose supplementation reduces the mortality rate and ICU admission rate of COVID-19 patients, while high-dose supplementation does not. However, in their classification of high and low doses, they arbitrarily assigned studies with individual doses of 200,000 IU and 400,000 IU to the high-dose group, while the remainders were categorized as low doses. They did not consider whether the low-dose group was administered continuously, nor did they compare the total intake of Vitamin D within a certain timeframe between the single high-dose and single low-dose groups. As a result, this approach failed to exclude the influence of factors such as continuous application or total intake on clinical outcomes. Our study is currently the only meta-analysis that simultaneously includes an analysis of both the method of Vitamin D intake and the total dosage within a specific period. Thus, the conclusions of this meta-analysis may facilitate the determination of more appropriate dosages and methods of Vitamin D supplementation, thereby achieving better clinical outcomes.
Previous studies have indicated that Vitamin D deficiency is associated with an increased risk of COVID-19 infection and poor outcomes (5, 10, 52, 62), Consequently, supplementing Vitamin D in deficient populations is more likely to improve COVID-19 prognosis, a finding supported by recent meta-analyses (63). However, few randomized clinical trials have directly grouped patients based on baseline Vitamin D concentrations. In our study, which included 21 clinical trials, 9 did not specify baseline Vitamin D levels, while the remaining 12 included only Vitamin D-deficient individuals (25OHD < 30 ng/mL). Our subgroup analysis using this criterion found that Vitamin D supplementation significantly improved mortality and ICU admission rates in deficient populations, along with a notable reduction in heterogeneity. In contrast, the group without baseline restrictions showed no significant effect on mortality and a significant effect on ICU admission rates, but with high heterogeneity. This discrepancy may be attributed to differences in baseline Vitamin D concentrations. Therefore, selecting appropriate patient populations for Vitamin D supplementation is crucial for optimizing its therapeutic efficacy.
Due to the significant variations in vitamin D supplementation doses and effects in existing clinical trials, there is currently no clear recommendation or consensus for the use of vitamin D specifically for COVID-19 patients. These studies have not provided explicit guidance on the specific applications and dosages of vitamin D in the treatment of COVID-19. As a result, most recommendations focus primarily on the preventive use of vitamin D against COVID-19, drawing on guidelines from other diseases. The main goal is to enhance immune function by maintaining adequate vitamin D levels. For instance, in 2020, international nutritional guidelines recommended a daily intake of 400 IU of vitamin D as a preventive measure against COVID-19, particularly for individuals with limited sun exposure (64, 65). However, in 2022, Griffin G conducted a critical review of the role of vitamin D in the prevention and treatment of diseases such as rickets, tuberculosis, and respiratory infections. He argued that a daily intake of 400 IU is insufficient to achieve optimal serum vitamin D levels, suggesting an increased daily dosage of 800–1,000 IU. For individuals suspected of having vitamin D deficiency, he recommended a higher initial dose of 4,000 IU per day for the first 4 weeks (55, 66). Our study investigated the effects of vitamin D supplementation in COVID-19 patients, focusing on baseline vitamin D levels, supplementation methods, and dosage. The findings indicate that vitamin D supplementation is significantly more effective in individuals with vitamin D deficiency, with optimal results observed when the total supplementation over 14 days is less than 100,000 IU. Although specific guidelines for vitamin D supplementation during COVID-19 are currently limited, our study is the first to demonstrate that a lower cumulative dose of less than 100,000 IU over 14 days is associated with better outcomes.
This study has certain limitations. Firstly, most of the included trials did not report the baseline blood concentrations of Vitamin D among participants, which limited our ability to accurately compare the supplementation effects between individuals with low and high baseline levels. Moreover, there was considerable variation across studies in terms of Vitamin D dosage, patient populations, and methods of administration. To address these differences and better capture real-world scenarios, we employed a random-effects model. Additionally, differences in baseline health conditions, illness severity, and concurrent medications among participants may have influenced the observed effects of Vitamin D supplementation. As this study did not perform stratified analyses based on these patient characteristics, future research should aim to explore these factors to provide a more comprehensive understanding.
5 Conclusion
The findings of this review support the conclusion that Vitamin D supplementation has a positive impact on the clinical outcomes of patients with COVID-19. Our analysis of the mode and dosage of Vitamin D supplementation indicates that continuous intake is associated with greater improvements in COVID-19 patients compared to single-dose treatments. Additionally, a total Vitamin D supplementation of less than 100,000 IU over 14 days is more effective than higher doses of 100,000 IU or more. Furthermore, Vitamin D supplementation shows significantly greater efficacy in individuals with Vitamin D deficiency. To further validate these findings, high-quality, long-term follow-up randomized controlled trials (RCTs) are necessary.
Data availability statement
The authors confirm that the data supporting the findings of this study are available in the article.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Conceptualization, Writing – review & editing. HD: Methodology, Writing – review & editing. NS: Investigation, Writing – review & editing. YL: Software, Writing – review & editing. YZ: Data curation, Writing – review & editing. SG: Conceptualization, Funding acquisition, Writing – review & editing. XM: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Key R&D Program of China (grant number: 2023YFC0872400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1441847/full#supplementary-material
References
1. Zdrenghea, MT, Makrinioti, H, Bagacean, C, Bush, A, Johnston, SL, and Stanciu, LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. (2017) 27:e1909. doi: 10.1002/rmv.1909
2. Vaghari-Tabari, M, Mohammadzadeh, I, Qujeq, D, Majidinia, M, Alemi, F, Younesi, S, et al. Vitamin D in respiratory viral infections: a key immune modulator? Crit Rev Food Sci Nutr. (2023) 63:2231–46. doi: 10.1080/10408398.2021.1972407
3. Fabbri, A, Infante, M, and Ricordi, C. Editorial - vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev Med Pharmacol Sci. (2020) 24:4048–52. doi: 10.26355/eurrev_202004_20876
4. Saraf, R, Jensen, BP, Camargo, CAJr, Morton, SMB, Jing, M, Sies, CW, et al. Vitamin D status at birth and acute respiratory infection hospitalisation during infancy. Paediatr Perinat Epidemiol. (2021) 35:540–8. doi: 10.1111/ppe.12755
5. Berry, DJ, Hesketh, K, Power, C, and Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. (2011) 106:1433–40. doi: 10.1017/S0007114511001991
6. Jolliffe, DA, Camargo, CAJr, Sluyter, JD, Aglipay, M, Aloia, JF, Ganmaa, D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. (2021) 9:276–92. doi: 10.1016/S2213-8587(21)00051-6
7. Vlieg-Boerstra, B, de Jong, N, Meyer, R, Agostoni, C, De Cosmi, V, Grimshaw, K, et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: a systematic review and meta-analysis. Allergy. (2022) 77:1373–88. doi: 10.1111/all.15136
8. Zhang, Y, Fang, F, Tang, J, Jia, L, Feng, Y, Xu, P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:l4673. doi: 10.1136/bmj.l4673
9. Annweiler, G, Corvaisier, M, Gautier, J, Dubée, V, Legrand, E, Sacco, G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. (2020) 12:3377. doi: 10.3390/nu12113377
10. Ben-Eltriki, M, Hopefl, R, Wright, JM, and Deb, S. Association between vitamin D status and risk of developing severe COVID-19 infection: a Meta-analysis of observational studies. J Am Nutr Assoc. (2022) 41:679–89. doi: 10.1080/07315724.2021.1951891
11. Dror, AA, Morozov, N, Daoud, A, Namir, Y, Yakir, O, Shachar, Y, et al. Pre-infection 25-hydroxyVitamin D3 levels and association with severity of COVID-19 illness. PLoS One. (2022) 17:e0263069. doi: 10.1371/journal.pone.0263069
12. Oscanoa, TJ, Amado, J, Vidal, X, Laird, E, Ghashut, RA, and Romero-Ortuno, R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyVitamin D concentration - a metaanalysis. Adv Respir Med. (2021) 89:145–57. doi: 10.5603/ARM.a2021.0037
13. Angelidi, AM, Belanger, MJ, Lorinsky, MK, Karamanis, D, Chamorro-Pareja, N, Ognibene, J, et al. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc. (2021) 96:875–86. doi: 10.1016/j.mayocp.2021.01.001
14. Sabico, S, Enani, MA, Sheshah, E, Aljohani, NJ, Aldisi, DA, Alotaibi, NH, et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid-19: a randomized clinical trial. Nutrients. (2021) 13:2170. doi: 10.3390/nu13072170
15. Ling, SF, Broad, E, Murphy, R, Pappachan, JM, Pardesi-Newton, S, Kong, MF, et al. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-Centre observational study. Nutrients. (2020) 12:3799. doi: 10.3390/nu12123799
16. Alcala-Diaz, JF, Limia-Perez, L, Gomez-Huelgas, R, Martin-Escalante, MD, Cortes-Rodriguez, B, Zambrana-Garcia, JL, et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. (2021) 13:1760. doi: 10.3390/nu13061760
17. Gonen, MS, Alaylioglu, M, Durcan, E, Ozdemir, Y, Sahin, S, Konukoglu, D, et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients. (2021) 13:12. doi: 10.3390/nu13114047
18. Cervero, M, Lopez-Wolf, D, Casado, G, Novella-Mena, M, Ryan-Murua, P, Taboada-Martinez, ML, et al. Beneficial effect of short-term supplementation of high dose of vitamin D(3) in hospitalized patients with COVID-19: a multicenter, single-blinded, prospective randomized pilot clinical trial. Front Pharmacol. (2022) 13:863587. doi: 10.3389/fphar.2022.863587
19. De Niet, S, Tremege, M, Coffiner, M, Rousseau, AF, Calmes, D, Frix, AN, et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: a randomized, double-blind, placebo-controlled trial. Nutrients. (2022) 14:3048. doi: 10.3390/nu14153048
20. Guven, M, and Gultekin, H. The effect of high-dose parenteral vitamin D(3) on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study. Eur J Clin Nutr. (2021) 75:1383–8. doi: 10.1038/s41430-021-00984-5
21. Maghbooli, Z, Sahraian, MA, Jamalimoghadamsiahkali, S, Asadi, A, Zarei, A, Zendehdel, A, et al. Treatment with 25-HydroxyVitamin D(3) (Calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: a pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr Pract. (2021) 27:1242–51. doi: 10.1016/j.eprac.2021.09.016
22. Rawat, D, Roy, A, Maitra, S, Shankar, V, Khanna, P, and Baidya, DK. Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr. (2021) 15:102189. doi: 10.1016/j.dsx.2021.102189
23. Cannata-Andía, JB, Díaz-Sottolano, A, Fernández, P, Palomo-Antequera, C, Herrero-Puente, P, Mouzo, R, et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. (2022) 20:83. doi: 10.1186/s12916-022-02290-8
24. Mariani, J, Antonietti, L, Tajer, C, Ferder, L, Inserra, F, Sanchez Cunto, M, et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: multicentre randomized controlled clinical trial. PLoS ONE. (2022) 17:e0267918. doi: 10.1371/journal.pone.0267918
25. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
26. Luo, D, Wan, X, Liu, J, and Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
27. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
28. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
29. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
30. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
31. Annweiler, C, Beaudenon, M, Gautier, J, Gonsard, J, Boucher, S, Chapelet, G, et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): a multicenter, open-label, randomized controlled superiority trial. PLoS Med. (2022) 19:e1003999. doi: 10.1371/journal.pmed.1003999
32. Bychinin, MV, Klypa, TV, Mandel, IA, Yusubalieva, GM, Baklaushev, VP, Kolyshkina, NA, et al. Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. Sci Rep. (2022) 12:18604. doi: 10.1038/s41598-022-22045-y
33. Dilokpattanamongkol, P, Yan, C, Jayanama, K, Ngamjanyaporn, P, Sungkanuparph, S, and Rotjanapan, P. Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: a single-center randomized controlled trial. BMC Complement Med Ther. (2024) 24:97. doi: 10.1186/s12906-024-04393-6
34. Domazet Bugarin, J, Dosenovic, S, Ilic, D, Delic, N, Saric, I, Ugrina, I, et al. Vitamin D supplementation and clinical outcomes in severe COVID-19 patients-randomized controlled trial. Nutrients. (2023) 15:1234. doi: 10.3390/nu15051234
35. Elamir, YM, Amir, H, Lim, S, Rana, YP, Lopez, CG, Feliciano, NV, et al. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. (2022) 154:116175. doi: 10.1016/j.bone.2021.116175
36. Entrenas Castillo, M, Entrenas Costa, LM, Vaquero Barrios, JM, Alcala Diaz, JF, Lopez Miranda, J, Bouillon, R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. (2020) 203:105751. doi: 10.1016/j.jsbmb.2020.105751
37. Fernandes, AL, Sales, LP, Santos, MD, Caparbo, VF, Murai, IH, and Pereira, RMR. Persistent or new symptoms 1 year after a single high dose of vitamin D(3) in patients with moderate to severe COVID-19. Front Nutr. (2022) 9:979667. doi: 10.3389/fnut.2022.979667
38. Fiore, V, De Vito, A, Bagella, P, Princic, E, Mariani, AA, Denti, L, et al. Effectiveness of vitamin D supplements among patients hospitalized for COVID-19: results from a monocentric matched-cohort study. Healthcare (Basel). (2022) 10:956. doi: 10.3390/healthcare10050956
39. Murai, IH, Fernandes, AL, Antonangelo, L, Gualano, B, and Pereira, RMR. Effect of a single high-dose vitamin D3 on the length of hospital stay of severely 25-HydroxyVitamin D-deficient patients with COVID-19. Clinics (São Paulo). (2021) 76:e3549. doi: 10.6061/clinics/2021/e3549
40. Nogues, X, Ovejero, D, Pineda-Moncusí, M, Bouillon, R, Arenas, D, Pascual, J, et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. (2021) 106:e4017–27. doi: 10.1210/clinem/dgab405
41. Sarhan, N, Abou Warda, AE, Sarhan, RM, Boshra, MS, Mostafa-Hedeab, G, ALruwaili, BF, et al. Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: A randomized clinical trial. Medicina (Kaunas). (2022) 58:1358. doi: 10.3390/medicina58101358
42. Singh, A, Rastogi, A, Puri, GD, Ganesh, V, Naik, NB, Kajal, K, et al. Therapeutic high-dose vitamin D for vitamin D-deficient severe COVID-19 disease: randomized, double-blind, placebo-controlled study (SHADE-S). J Public Health (Oxf). (2024) 46:256–66. doi: 10.1093/pubmed/fdae007
43. Shah, K, Saxena, D, and Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM. (2021) 114:175–81. doi: 10.1093/qjmed/hcab009
44. Beran, A, Mhanna, M, Srour, O, Ayesh, H, Stewart, JM, Hjouj, M, et al. Clinical significance of micronutrient supplements in patients with coronavirus disease 2019: a comprehensive systematic review and meta-analysis. Clin Nutr ESPEN. (2022) 48:167–77. doi: 10.1016/j.clnesp.2021.12.033
45. Kümmel, LS, Krumbein, H, Fragkou, PC, Hünerbein, BL, Reiter, R, Papathanasiou, KA, et al. Vitamin D supplementation for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Front Immunol. (2022) 13:1023903. doi: 10.3389/fimmu.2022.1023903
46. Szarpak, L, Filipiak, KJ, Gasecka, A, Gawel, W, Koziel, D, Jaguszewski, MJ, et al. Vitamin D supplementation to treat SARS-CoV-2 positive patients. Evidence from meta-analysis. Cardiol J. (2022) 29:188–96. doi: 10.5603/CJ.a2021.0122
47. Tentolouris, N, Samakidou, G, Eleftheriadou, I, Tentolouris, A, and Jude, EB. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev. (2022) 38:e3517. doi: 10.1002/dmrr.3517
48. Chen, J, Mei, K, Xie, L, Yuan, P, Ma, J, Yu, P, et al. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: a meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr J. (2021) 20:89. doi: 10.1186/s12937-021-00744-y
49. Zaazouee, MS, Eleisawy, M, Abdalalaziz, AM, Elhady, MM, Ali, OA, Abdelbari, TM, et al. Hospital and laboratory outcomes of patients with COVID-19 who received vitamin D supplementation: a systematic review and meta-analysis of randomized controlled trials. Naunyn Schmiedeberg's Arch Pharmacol. (2023) 396:607–20. doi: 10.1007/s00210-022-02360-x
50. Sartini, M, Del Puente, F, Oliva, M, Carbone, A, Bobbio, N, Schinca, E, et al. Preventive vitamin D supplementation and risk for COVID-19 infection: a systematic review and Meta-analysis. Nutrients. (2024) 16:679. doi: 10.3390/nu16050679
51. Sîrbu, AC, Sabin, O, Bocșan, IC, Vesa, ȘC, and Buzoianu, AD. The effect of vitamin D supplementation on the length of hospitalisation, intensive care unit admission, and mortality in COVID-19-a systematic review and Meta-analysis. Nutrients. (2023) 15:3470. doi: 10.3390/nu15153470
52. D'Ecclesiis, O, Gavioli, C, Martinoli, C, Raimondi, S, Chiocca, S, Miccolo, C, et al. Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS One. (2022) 17:e0268396. doi: 10.1371/journal.pone.0268396
53. Hosseini, B, El Abd, A, and Ducharme, FM. Effects of vitamin D supplementation on COVID-19 related outcomes: a systematic review and meta-analysis. Nutrients. (2022) 14:2134. doi: 10.3390/nu14102134
54. Martineau, AR, Jolliffe, DA, Hooper, RL, Greenberg, L, Aloia, JF, Bergman, P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017) 356:i6583. doi: 10.1136/bmj.i6583
55. Griffin, G, Hewison, M, Hopkin, J, Kenny, RA, Quinton, R, Rhodes, J, et al. Perspective: vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: implications for COVID-19. Clin Med (Lond). (2021) 21:e144–9. doi: 10.7861/clinmed.2021-0035
56. Feiner Solís, Á, Avedillo Salas, A, Luesma Bartolomé, MJ, and Santander Ballestín, S. The effects of vitamin D supplementation in COVID-19 patients: a systematic review. Int J Mol Sci. (2022) 23:12424. doi: 10.3390/ijms232012424
57. Ketha, H, Thacher, TD, Oberhelman, SS, Fischer, PR, Singh, RJ, and Kumar, R. Comparison of the effect of daily versus bolus dose maternal vitamin D(3) supplementation on the 24,25-dihydroxyVitamin D(3) to 25-hydroxyVitamin D(3) ratio. Bone. (2018) 110:321–5. doi: 10.1016/j.bone.2018.02.024
58. Kundu, R, Chain, BM, Coussens, AK, Khoo, B, and Noursadeghi, M. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur J Immunol. (2014) 44:1781–90. doi: 10.1002/eji.201344157
59. Wall-Gremstrup, G, Holt, R, Yahyavi, SK, Jorsal, MJ, Juul, A, Jørgensen, N, et al. High-dose vitamin D(3) supplementation shows no beneficial effects on white blood cell counts, acute phase reactants, or frequency of respiratory infections. Respir Res. (2024) 25:11. doi: 10.1186/s12931-023-02642-9
60. Vieth, R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res. (2009) 29:3675–84.
61. Bacchetta, J, Sea, JL, Chun, RF, Lisse, TS, Wesseling-Perry, K, Gales, B, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyVitamin D in human monocytes. J Bone Miner Res. (2013) 28:46–55. doi: 10.1002/jbmr.1740
62. Coudray, MS, Hansel, S, Alesci, S, Meyer, WA3rd, Christenson, RH, Landry, LG, et al. Vitamin D levels and SARS-CoV-2 infection among medically underserved populations in the minority and rural coronavirus insights study. Viruses. (2024) 16:639. doi: 10.3390/v16040639
63. Meng, J, Li, X, Liu, W, Xiao, Y, Tang, H, Wu, Y, et al. The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: a meta-analysis of randomized controlled trials. Clin Nutr. (2023) 42:2198–206. doi: 10.1016/j.clnu.2023.09.008
64. Grant, WB, Al Anouti, F, Boucher, BJ, Dursun, E, Gezen-Ak, D, Jude, EB, et al. A narrative review of the evidence for variations in serum 25-HydroxyVitamin D concentration thresholds for optimal health. Nutrients. (2022) 14:639. doi: 10.3390/nu14030639
65. Cawood, AL, Walters, ER, Smith, TR, Sipaul, RH, and Stratton, RJ. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients. (2020) 12:3230. doi: 10.3390/nu12113230
Keywords: COVID-19, vitamin D, prognosis, administration methods, dosage, baseline vitamin D
Citation: Zhang X, Wu J, Dong H, Shang N, Li Y, Zhang Y, Guo S and Mei X (2024) The impact of supplementing vitamin D through different methods on the prognosis of COVID-19 patients: a systematic review and meta-analysis. Front. Nutr. 11:1441847. doi: 10.3389/fnut.2024.1441847
Edited by:
Shuang Song, Dalian Polytechnic University, ChinaReviewed by:
Seyed-Amir Tabatabaeizadeh, Islamic Azad University of Mashhad, IranLeigh A. Frame, George Washington University, United States
Copyright © 2024 Zhang, Wu, Dong, Shang, Li, Zhang, Guo and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shubin Guo, c2h1YmluMDA3QHllYWgubmV0; Xue Mei, bWVpeHVlOTFAeWVhaC5uZXQ=
†These authors have contributed equally to this work
 Xiangqun Zhang
Xiangqun Zhang Junyuan Wu1,2
Junyuan Wu1,2 Xue Mei
Xue Mei