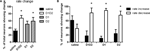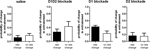1
Section on In Vivo Neural Function, Laboratory for Integrative Neuroscience, National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, Bethesda, MD, USA
2
Champalimaud Neuroscience Programme at Instituto Gulbenkian de Ciência, Oeiras, Portugal
Previous studies showed that dopamine depletion leads to both changes in firing rate and in neuronal synchrony in the basal ganglia. Since dopamine D1 and D2 receptors are preferentially expressed in striatonigral and striatopallidal medium spiny neurons, respectively, we investigated the relative contribution of lack of D1 and/or D2-type receptor activation to the changes in striatal firing rate and synchrony observed after dopamine depletion. Similar to what was observed after dopamine depletion, co-administration of D1 and D2 antagonists to mice chronically implanted with multielectrode arrays in the striatum caused significant changes in firing rate, power of the local field potential (LFP) oscillations, and synchrony measured by the entrainment of neurons to striatal local field potentials. However, although blockade of either D1 or D2 type receptors produced similarly severe akinesia, the effects on neural activity differed. Blockade of D2 receptors affected the firing rate of medium spiny neurons and the power of the LFP oscillations substantially, but it did not affect synchrony to the same extent. In contrast, D1 blockade affected synchrony dramatically, but had less substantial effects on firing rate and LFP power. Furthermore, there was no consistent relation between neurons changing firing rate and changing LFP entrainment after dopamine blockade. Our results suggest that the changes in rate and entrainment to the LFP observed in medium spiny neurons after dopamine depletion are somewhat dissociable, and that lack of D1- or D2-type receptor activation can exert independent yet interactive pathological effects during the progression of Parkinson’s disease.
The basal ganglia are known to be involved in action selection and movement initiation (Groenewegen, 2003
; Gurney et al., 2004
). Dopamine (DA) transmission within the basal ganglia is essential for the normal expression of spontaneous and voluntary movement (Poirier et al., 1975
; Amalric and Koob, 1987
; Fletcher and Starr, 1987
; Zhou and Palmiter, 1995
). Dysfunction of DA transmission has profound consequences upon the function of the basal ganglia, altering downstream activity and motor output (Lloyd, 1977
; Filion, 1979
; Sanderson et al., 1986
; Pan and Walters, 1988
; MacLeod et al., 1990
; Calabresi et al., 1993
; Burbaud et al., 1995
; Chesselet and Delfs, 1996
; Levy et al., 1997
; Murer et al., 1997
; Rohlfs et al., 1997
; Moore et al., 1998
; Ni et al., 2000
; Chen et al., 2001
; Magill et al., 2001
; West and Grace, 2002
). Loss of DA projections is the characteristic morphological feature of Parkinson’s disease (PD) (Shimohama et al., 2003
), wherein degeneration of substantia nigra pars compacta (SNc) projections results in decreased extracellular striatal DA levels (Schober, 2004
). These changes in dopamine levels lead to changes in striatal firing rate, and it is generally believed that upon DA depletion there is increased activity of indirect pathway neurons (striatopallidal neurons, which express mainly D2 type receptors) and decreased activity of direct pathway neurons (striatonigral neurons, which express predominantly D1 type receptors), ultimately resulting in inhibition of motor cortex activity (Albin et al., 1989
; Alexander and Crutcher, 1990
; Jenner, 1995
). However, several recent studies failed to observe overall decrease in firing rate in motor cortex after dopamine depletion (Goldberg et al., 2004
; Costa et al., 2006
), even when pronounced changes in striatal firing rate were observed (Costa et al., 2006
).
It is also increasingly evident that DA loss can result in abnormal oscillatory activity and increased synchrony in the basal ganglia. Slice studies, pharmacological and lesion models of PD in rodents and primates, and studies of idiopathic PD patients have shown the appearance and possible propagation of rhythmic and synchronous firing in basal ganglia structures. Organotypic cultures of the rat external globus pallidus (GPe)-subthalamic nucleus (STN) network have suggested the existence of a basal ganglia pacemaker center in the absence of DA (Plenz and Kital, 1999
). Dual site single-unit studies in lesioned primates and human PD patients have shown correlated oscillatory firing between basal ganglia neuron pairs (Bergman et al., 1994
; Levy et al., 2000
; Raz et al., 2000
, 2001
; Brown et al., 2001
, 2004
; Brown, 2003
). Neuronal population correlates of PD and related DA dysfunctions in awake behaving animals have been less studied, but some recent results suggest that acute DA depletion or DA receptor blockade results in synchronous and oscillatory activity across basal ganglia neuron populations (Costa et al., 2006
; Burkhardt et al., 2007
), even when no net change in motor cortex firing rate is observed (Costa et al., 2006
). Additionally, local field potential (LFP) recordings show decreased power in the gamma frequencies (30–60 Hz) and increased power of lower frequency oscillations, namely in the beta band (10–30 Hz), in the subthalamic nucleus, cortex, and dorsal striatum (Kuhn et al., 2005
; Sharott et al., 2005
; Costa et al., 2006
; Androulidakis et al., 2008
; Mallet et al., 2008
) after dopamine depletion. It has been postulated that this oscillatory activity is pathological, and possibly a neurological correlate of Parkinsonian motor deficits (Bevan et al., 2002
; Hammond et al., 2007
).
Although it is clear from the studies mentioned previously that DA depletion can lead to both changes in firing rate and in synchrony in striatum (e.g. Costa et al., 2006
), it is not known if these changes are mechanistically related or independent. For example, although it is known that DA D1–type receptors (D1) and DA D2–type receptors (D2) have different physiological properties and different distributions in striatal neural populations (Gerfen et al., 1990
; Joyce, 1993
; Surmeier and Kitai, 1994
; Wooten, 1997
; Bertran-Gonzalez et al., 2008
; Taverna et al., 2008
), it is not known if lack of D1 or D2 type receptor activation produces similar effects in striatal rate and synchrony. Furthermore, there may be more interactions between the direct and indirect pathways than initially thought (Lévesque and Parent, 2005
; Nadjar et al., 2006
; Taverna et al., 2008
), and different receptors of each receptor subtype could be co-expressed and have different functions within the same neuron (Fiorentini et al., 2008
; Marcellino et al., 2008
). It is therefore important to investigate if striatal changes in rate and synchrony upon DA depletion are related.
In this study, we investigated the effects of D1 and D2 receptor antagonism on the firing rate and synchrony of striatal neurons in awake behaving mice, by recording the activity from multiple single units and local field potential oscillations in the dorsal striatum before and after acute dopamine receptor blockade with SCH-23390 (a D1-type antagonist), raclopride (a D2-type antagonist), or both. As observed after acute DA depletion (Costa et al., 2006
), D1 + D2 receptor blockade caused (1) changes in firing rate with the majority of medium spiny neurons decreasing firing frequency, (2) changes in the relative power of the LFP oscillations in striatum, and (3) increase in entrainment of medium spiny neurons to the LFP. However, although blockade of D1 or D2 receptors alone produced a similarly profound akinesia, the effects of D1 or D2 antagonism on striatal firing rate and synchrony, measured by entrainment to the local field potential oscillations, were different. Blockade of D2-type receptors affected the firing rate of medium spiny neurons and the power of the LFP oscillations substantially, but did not affect synchrony, while D1 blockade affected synchrony dramatically. We failed to observe a consistent relation between a neuron changing firing rate and changing LFP entrainment after DA-receptor blockade. These results suggest that lack of D1 and D2 type receptor activation can exert independent yet interactive effects, which may interact in PD.
Animals
All experiments were approved by the NIAAA ACUC. Subjects were experimentally naïve, adult male C57BL/6J mice purchased from the Jackson laboratory, initially weighing 25–30 g, ages 3–6 months. To minimize the number of animals used and allow comparisons in the same individual, all subjects received all the treatments using a latin square design, which controls for order and carry-over effects. Subjects were given 1–3 days between sessions to allow for complete recovery from injections. Twenty-nine mice were used for behavioral testing, and a total of 14 subjects were implanted for multielectrode recording. Two subjects lost their microelectrode headstages prior to completion of all treatment conditions and had to be euthanized; only the data from completed sessions are included in our analyses.
Animals were housed under a 12-h dark-light cycle (lights off at 19:00). Experimental procedures were performed during the light phase of their cycle. Animals had free access to food and water at all times except during recording sessions.
Drugs
We used the DA D1-receptor antagonist SCH-23390 (Sigma, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1 H-3-benzazepine), and the DA D2-receptor antagonist raclopride (Sigma, 3,5-dichloro-N-(1-ethylpyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxybenzamide). All drugs were dissolved in phosphate-buffered saline with 1% DMSO, which also served as the control injection. Injections were given intra-peritoneally (i.p.) at 10 mL/kg. As previously stated, in both locomotor assessment and electrophysiological recordings, the treatments (saline, SCH-23390, raclopride, and SCH-23390 + raclopride) were administered to the same subjects using a latin square design. Different groups of animals were used for the administration of different doses. For the D1 blockade sessions, SCH-23390 was administered at 0.4 mg/kg. For D2 sessions, raclopride was administered at 2.0 mg/kg. For D1 + D2 sessions, SCH-23390 and raclopride were co-administered at these same doses. These doses were selected because co-administration at these doses blocks completely the effects of L-DOPA after DA-depletion (Costa et al., 2006
).
Locomotor Assessment
Spontaneous locomotor activity was measured using infrared beams (Opto M3, Columbus Instruments, Columbus, OH, USA). Prior to assessment, subjects were randomly assigned to either a drug or control group. Subjects were placed in a novel cage, and ambulatory counts were recorded for 30 min under dim illumination. After 30 min, subjects received either i.p. drug or saline vehicle injections, and were placed in a second novel cage where ambulatory counts were recorded for 60 min. Ambulatory counts were tallied for each 5-min bin for subsequent analysis.
Surgery
Animals were anesthetized with isofluorane and placed in a stereotaxic apparatus. The scalp was shaved and swabbed with iodine. A central incision was made to expose the skull. The skull was mapped stereotaxically for bregma, and two craniotomies approximately 1 mm wide and 2 mm long were drilled in the skull bilaterally (centered at +0.5 mm AP, ±1.8 mm ML, −2.3 mm DV; all coordinates relative to bregma). A 16-microelectrode array designed to target dorsal striatum was lowered into each hemisphere. Neural activity was monitored online while lowering electrodes into the striatum to ensure proper electrode depth and positioning. Ground wires were wrapped around skull screws, and the microelectrode arrays were anchored with dental acrylic, using the skull screws as anchors. Subjects were given at least 5 days to recover after surgery before beginning and experimental procedures.
Microarrays
Electrode microarrays were obtained from CD Neural Technologies (Durham, NC, USA). The arrays were configured in two rows of eight microelectrodes, with 1000 μm of space between rows and 200 μm of space between electrodes within a row. Microelectrodes consisted of 50 μm tungsten wire with platinum-plated tips connected to a printed circuit board (PCB). The PCB was attached to a connector plug on the opposing side, for connection to the recording preamplifier (Costa et al., 2004
).
Neuronal and LFP Recordings
Single-unit and LFP activity were recorded using the MAP system (Plexon Inc, TX, USA) using established procedures (Costa et al., 2006
). Subsequent to recording, units were further sorted and clarified offline via Offline Sorter (Plexon Inc, TX, USA). In the saline condition, 147 units were recorded (13.4 ± 2.0 per subject, n = 11). During the D1 + D2 sessions, 99 units were recorded (14.1 ± 1.8, n = 7). In the D1 condition, 140 units were recorded (15.6 ± 1.4, n = 9). In the D2 condition, 138 units were recorded (17.3 ± 3.4, n = 8). For clarification and differentiation of neuron type, single-units were sorted and exported to Matlab (MathWorks, Natick, MA, USA). To separate between putative medium spiny neurons (MSNs), fast-spiking interneurons (FSIs), and large aspiny cholinergic interneurons (LANs), we calculated the firing rate during the first 25 min after putting the animal into the recording cage (before any drug injection); the amplitude of the spikes as the maximal peak-valley difference for each neuronal waveform; and the half-width of each waveform as the valley width at the half-maximum of spike amplitude. Although extracellular recordings do not allow for definitive classification of neuronal types, putative fast-spike interneurons were identified as waveform half-width less than 150 μs and baseline firing rate more than 10 Hz. Putative cholinergic interneurons were identified as tonically active with half-width of spike waveform more than 400 μs. The rest were treated as putative medium spiny projection neurons in striatum.
Data Analysis
Data analyses were carried out using the Neuroexplorer (Nex Technologies) and Matlab software packages.
Firing rate
To determine whether a neuron showed a significant change in firing rate subsequent to injection, we calculated the average firing frequency in 100-s bins for 900 s before and after the injection time, and pre-injection bins were compared against post-injection bins using a paired t-test (α = 0.01). We considered a neuron to show a significant change in firing rate if this comparison proved significant.
Spike-triggered average
An STA was considered to be significant if 20 consecutive 1 ms bins between −100 and +100 ms from 0 passed either above or below the maximal or minimal values observed in the periods between −3000 to −1000 ms and +1000 to +3000 ms from 0 (Costa et al., 2006
).
LFP power spectrum
The power spectrum of LFP was estimated for each 2-s sliding window with 1-s step via Welch’s method, and the parameters were chosen to allow for a frequency resolution of 0.5 Hz.
Power spectrum index
To analyze the changes in the relative power of different frequency oscillations across different states, we calculated a power spectrum index (PSI) as the average power at 4.5–9 Hz * 30–55 Hz/1.5–4 Hz * 11–30 Hz (Costa et al., 2006
).
Statistics
Statistical comparisons were performed using the SPSS (SPSS, Chicago, IL, USA) software package. Unless otherwise noted, all results were computed and averaged per subject, and subsequent statistical analyses were performed on each subject’s average value.
Locomotor sessions were analyzed via repeated-measures ANOVA, using Fisher’s LSD as a post-hoc measure (α = 0.05). Changes in firing rate and entrainment-rate interactions were analyzed using single-factor ANOVA to examine main effects, using Fisher’s LSD as a post-hoc measure (α = 0.05). For pre-post comparisons (e.g. LFP entrainment, and PSI comparisons) we used a paired t-test (α = 0.05). When performed, direct comparisons between specific experiments (e.g. D1 vs. D2) were performed via unpaired t-test (α = 0.05).
All data are presented as mean and standard error of the mean (SEM).
Acute Dopamine Receptor Blockade Produces Profound Akinesia
We first examined how acute blockade of D1-type, D2-type, or both types of DA receptors would affect spontaneous locomotion. Mice were allowed to move freely within a novel cage, and ambulatory counts were assessed for pre- and post-drug conditions.
We observed a main effect of treatment, with all treatments producing a significant decrease in spontaneous locomotion in comparison to saline injection (F3,83 = 28.09, p < 0.05; Figure 1
). Notably, concurrent blockade of D1 and D2 receptor types produced almost complete akinesia through the first 45 min of the sessions (F1,27 = 27.99, p < 0.05) (Figure 1
A). Blockade of either D1 (F1,27 = 42.02, p < 0.05) or D2 (F1,27 = 43.16, p < 0.05) type receptors alone (Figures 1
B,C, respectively) produced severe akinesia to a similar extent, though of lesser magnitude than the akinesia produced by concurrent blockade of both receptor types. There was a significant effect of the different DA treatments used (F2,41 = 8.78, p < 0.05); D1 + D2-receptor blockade differed significantly from both D1-receptor blockade (p < 0.05) and D2-receptor blockade (p < 0.05), but D1-receptor blockade and D2-receptor blockade were not statistically different (p > 0.05).
Figure 1. Effect of dopamine receptor blockade on spontaneous locomotion. (A) D1 + D2 antagonist. (B) D1 antagonist. (C) D2 antagonist. All treatments significantly reduced spontaneous locomotion in comparison to saline injection. Effect of combined D1 + D2 antagonist treatment was significantly greater than either D1 antagonist alone or D2 antagonist alone.
Effects of Dopamine Receptor Blockade on Striatal Neuron Firing Rate
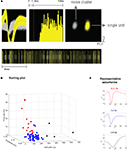
We investigated the effects of DA-receptor blockade on striatal neuronal activity by continuously recording the activity of the multiple single units and LFPs in the dorsal striatum. Neurons were isolated from background noise on the basis of waveform, interspike interval, principle component clustering, and firing pattern (Figure 2
A), and we followed the continuous activity of each neuron during the whole experimental session, with 30 min recorded pre-injection and 60 min recorded post-injection. Putative medium spiny neurons (MSNs) were discriminated from fast-spiking interneurons (FSIs) and large aspiny neurons (LANs) on the basis of half-width, firing frequency, and amplitude (Figures 2
B,C). Because of the low number of interneurons recorded, all analyses reported here were performed using putative MSNs.
Although intraperitoneal injections are not ideal to study the changes in local circuit function because they affect DA receptors widely, they permit us to follow the same neurons before and after injection, which is very difficult to achieve using local injection of the antagonists due to the pressure applied to the tissue around the electrodes. Nonetheless, in PD, loss of DA function is not localized to the striatum, and at the doses used here, the blockade of D1 and D2 type receptors in the basal ganglia and their inputs should be complete, and therefore most effects observed should result from changes in dopamine receptor activation in cortico-basal ganglia circuits.
Figure 2. (A) Example of a sorted single unit, recorded 2 weeks subsequent to electrode implantation. From left: unit waveforms (yellow) isolated from noise (gray) (x-axis, 800 μs; y-axis, 120 μV); interspike interval histogram; isolated unit and noise on a principle component plot (x-axis, PC1; y-axis, PC2); bottom, raster trace of unit events and noise events over a selected time period. (B,C) Sorting criteria for identifying putative medium spiny neurons (MSNs), fast-spiking interneurons (FSIs), and large aspiny neurons (LANs). (B) Criteria used for identifying putative neuron types were half-width (x-axis), firing rate (y-axis), and amplitude (z-axis). (C) Representative waveforms. Top panel (in red) depicts a putative FSI. Middle panel (in blue) depicts a putative MSN. Bottom panel (in black) depicts a putative LAN.
It has been previously shown that acute DA depletion causes a large percentage of striatal medium spiny neurons to change their firing rate, and that most of these neurons decrease their firing rate after DA depletion (Costa et al., 2006
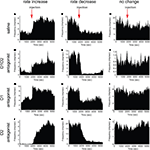
). We therefore investigated the effects of acute dopamine blockade on striatal firing rate. We observed that in each treatment condition neurons showed increase, decrease, or no change in firing rate (examples shown in Figures 3
A–L). There was a main effect of treatment (F3,34 = 3.93, p < 0.05), and similarly to what we observed during acute dopamine blockade, we found that the majority of striatal neurons displayed a significant change in firing rate in response to D1 + D2 blockade (67.5%), as compared to saline (31.9%) (p < 0.05). Blockade of D2 type receptors alone also caused a significant proportion of neurons to change firing rate (59.3%) compared to saline (p < 0.05), while blockade of D1-type receptors did not (50.8%) (p > 0.05) (Figure 4
A, although direct comparison of the magnitude of the effects after D1 and D2 treatments showed no significant difference between them, T5 = 0.30, p > 0.05).
Figure 3. Examples of firing rate change in single neurons. Single neurons showing increase (left column) and decrease (center column) in firing rate were observed after all treatment conditions. Neurons displaying no significant change in rate were also observed (right column). (A–C) Saline injection. (D–F) D1 + D2 antagonist injection. (G–I) D1 antagonist injection. (J–L) D2 antagonist injection.
Figure 4. Effect of dopamine receptor blockade on neuronal firing rate. (A) Percentage of total neurons showing a significant change in firing rate after treatment. After D1 + D2 and D2 receptor blockade more neurons changed firing rate than after saline injection. (B) Proportion of the neurons that changed firing rate showing increase in firing rate versus decrease in firing rate. With saline injection the proportion of neurons showing rate increase versus decrease was equivalent to each other. With all dopamine receptor antagonist treatments, significantly more neurons showed decrease in firing rate (∼80%) than increase in rate.
We next investigated if the changes in firing rate corresponded to increases or decreases in firing frequency. In the saline condition, a similar proportion of neurons increased (61.58 ± 12.40%) versus decreased (38.42 ± 12.40%) firing rate (F1,19 = 1.75, p > 0.05; Figure 4
B). However, after D1 + D2 blockade, the majority of neurons decreased firing (82.25 ± 13.88% versus 17.75 ± 13.88% increasing, F1,13 = 10.80, p < 0.05), as it was observed after acute DA depletion (Costa et al., 2006
). Similar effects were observed after D1 (89.09 ± 5.84% decreasing versus 10.91 ± 5.84% increasing, F1,17 = 89.74, p < 0.05) and surprisingly, also after D2 blockade (75.84 ± 11.50% increasing versus 24.16 ± 11.50% decreasing, F1,15 = 10.10, p < 0.05). There were no significant differences observed among the different DA-blockade treatments (p < 0.05), so the difference observed in the number of neurons changing rate after D1 or D2 blockade cannot be attributed to differences in the sign of the changes (increase versus decrease of firing rate).
Taken together, these data suggest that D2-receptor blockade produces similar changes in medium spiny neuron firing rate in the dorsal striatum to those caused by D1 + D2-receptor blockade, while complete blockade of D1-receptors produces smaller changes.
However, the majority of neurons changing firing rate after D1 blockade decreased firing rate suggesting that D1-receptor blockade produces similar changes in rate to D2-receptor blockade, but in a smaller population of neurons.
Differential Effects of D1 and D2 Acute Dopamine Blockade on Striatal LFP Power
Our previous results showed that acute DA-depletion causes profound changes in the power of the striatal local field potential (LFP) oscillations, with mainly gamma oscillations decreasing in power, while beta and delta oscillations increased in power (Costa et al., 2006
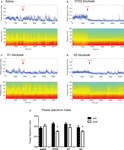
). This was evident by a decrease in the ratio between the power of gamma and theta oscillations, over the power of beta and delta oscillations (4.5–9 Hz * 30–55 Hz/1.5–4 Hz * 11–30 Hz, herein designed as power spectrum index or PSI). We therefore examined the relative changes in the power of LFP oscillations after the different conditions of acute dopamine blockade (examples shown in Figure 5
). D1 + D2-receptor blockade (Figure 5
B) resulted in a visible decrease in power of gamma range (30–55 Hz) oscillations and an increase in power of lower frequency oscillations, e.g. beta (11–30 Hz), an effect not seen with saline injection (Figure 5
A).
Figure 5. (A–D) Examples of LFP power spectra and corresponding power spectrum index for different treatments throughout the time course of the experiment. Arrow indicates the time of injection. (E) Summary of the effect of dopamine receptor blockade on power spectrum index. D1 + D2 antagonist and D2 antagonist caused a significant decrease in power spectrum index after injection.
The investigation of the relative changes in the power of LFP oscillations revealed that D1 + D2-receptor blockade produced a robust decrease in PSI (T4 = 4.86, p < 0.05, Figure 4
E). Blockade of D2-receptors likewise resulted in a significant decrease in PSI (T4 = 3.34, p < 0.05). In contrast, we observed no decrease in PSI after D1-receptor blockade (T4 = 1.36, p > 0.05), or saline injection (T5 = −1.11, p > 0.05).
These results indicate that, at these doses, D2-receptor blockade has greater influence in the relative power of the LFP oscillations than D1-receptor blockade. It is interesting that the treatments that had a greater effect in firing rate were also the treatments that decreased the relative power of gamma oscillations (D1 + D2 and D2 blockade), especially given recent studies which have associated changes in interneuron firing to both gamma oscillations and firing rate (Sohal et al., 2009
).
Effects of Dopamine Receptor Blockade on Striatal Ensemble Coordination
As previously mentioned, dopamine depletion does not only cause changes in firing rate and LFP power, but also in the coordinated activity of neurons, as measured for example by changes in the entrainment of neurons to the LFP (Costa et al., 2006
). We therefore examined how acute DA-blockade changed the entrainment of medium spiny neurons to LFP oscillations by calculating the spike triggered averages (STAs) of the LFPs (Fries et al., 2001
; Goldberg et al., 2004
; Kuhn et al., 2005
; Costa et al., 2006
).
Consistent with previous results, all conditions showed a baseline entrainment in ∼20–30% of neurons. As expected, we observed no significant increase in entrainment to the LFP post-injection in the saline condition (T6 = −2.16, p > 0.05, Figures 6
B,E), and we observed a significant increase in entrainment after D1 + D2 blockade (T4 = −3.14, p < 0.05), and effect which was significantly different from saline (p < 0.05). However, in contrast to the effects on rate, we found that D1 blockade produced a significant increase in entrainment subsequent to injection (T5 = −3.14, p < 0.05), while D2 receptor blockade did not (T3 = −0.93, p > 0.05).
Figure 6. (A–D) Representative spike-triggered average histograms. (A) An MSN shows no significant entrainment to the LFP in the baseline state, but develops a pronounced entrainment to the trough of the LFP oscillation subsequent to DA receptor blockade. (B) An MSN showing no entrainment to the LFP continues to show no entrainment after saline injection. (C) An FSI shows some entrainment to the LFP peak in the pre-injection state, with a substantial increase in entrainment post-injection. (D) A LAN shows significant entrainment to the LFP trough in both pre- and post-injection. (E) D1 + D2 antagonists and D1 antagonist alone resulted in a significant increase in the percentage of neurons showing entrainment to the LFP oscillation post-injection, while D2 antagonist alone and saline produced no significant changes.
Interestingly, putative MSNs showing entrainment to the LFP tended to fire around or after the trough of the extracellular recorded LFP oscillation after DA-blockade, which corresponds to the point of highest intracellular depolarization (representative STA shown in Figure 6
A). In contrast, FSIs fired preferentially at the peak of the LFP, when intracellular potentials should be more hyperpolarized (consistent with inhibition being maximal at this point; Figure 6
C), while putative LANs tended to fire after the trough of the LFP oscillation (Figure 6
D).
Taken together, these data confirm that acute D1 + D2 receptor blockade leads to increased entrainment of striatal medium spiny neurons to the LFP as observed after DA depletion (Costa et al., 2006
). Interestingly, blockade of D1 receptors alone caused a similar increase in entrainment to the LFP, while blockade of D2 receptors did not.
Relation Between the Changes in Firing Rate and LFP Entrainment after DA-Blockade
The effects of the different dopamine treatments described above suggest that the influences DA signaling on striatal firing rate and synchrony are somewhat dissociable. However, this evidence is indirect and based solely on the different magnitudes of the effects of D1 and D2 receptor blockade. We therefore investigated whether there was any relation between the probability of a neuron changing firing rate and changing entrainment to the LFP after DA-blockade. We examined in each treatment condition whether neurons showing a significant change in firing rate or no change in firing rate would be more or less likely to show a significant change in LFP entrainment (Figure 7
). We observed no significant differences in the probability of a change in entrainment between rate-changing neurons and non-rate-changing neurons (F1,13 = 0.56, F1,9 = 0.87, F1,11 = 3.39, F1,7 = 0.01 for saline, D1 + D2, D1, and D2 conditions, respectively; all p > 0.05), suggesting that there was no consistent relation between changes in firing rate and changes in entrainment after DA-blockade.
Figure 7. Probability of changing entrainment to the LFP for neurons changing or not changing firing rate after DA-blockade. Across all treatment conditions there was no difference in the probability of changing entrainment to the LFP between neurons that changed firing rate versus neurons that did not change firing rate after DA-blockade.
The goal of this study was to investigate the effects of selective acute blockade dopamine D1-type receptors and D2-type receptors on the activity of neuronal ensembles in the dorsal striatum. We found that acute concurrent blockade of D1 and D2 receptors produced complete akinesia, while blockade of D1- or of D2-type DA receptors alone resulted in lower but similar levels of akinesia. Concurrent blockade of D1 and D2 receptors caused significant changes in striatal neural activity and synchrony, and recapitulated the effects observed after acute dopamine depletion (Costa et al., 2006
). In summary, after acute D1 + D2 blockade, the majority of neurons in the striatum decreased firing rate, the power of the local field potential changed with the power of gamma oscillations decreasing while the power of beta and delta oscillations increased, and significantly more neurons became entrained to the LFP. Nonetheless, although in the results presented here acute dopamine receptor blockade in awake behaving mice seems to produce decreased firing rate and increased synchrony and beta oscillations, it is important to note that exaggerated pathological changes in firing rate, oscillatory activity, and synchrony observed in the basal ganglia of Parkinson’s patients may take longer to develop, and be the result of more chronic plastic changes in basal ganglia circuits (Liang et al., 2008
; Mallet et al., 2008
).
Interestingly, although blockade of either D1 or D2 alone was sufficient to cause similarly profound locomotor effects, the effects of D1 blockade alone and D2 blockade alone on striatal neural activity differed substantially. Blockade of D2 receptors resulted in a dramatic change in the firing rate of the majority of the medium spiny neurons recorded, and in a change in the relative power of the local field potential oscillations, but did not cause substantial changes in synchrony as measured by entrainment of the neural activity to the LFP oscillations. In contrast, D1 receptor blockade caused substantial changes in synchrony but had less effect on firing rate and power of the LFP. Together with the lack of relation between a neuron changing firing rate and changing LFP entrainment after DA-blockade, these results suggest that the effects of DA-depletion on striatal rate and on synchrony may be dissociable.
Since both D1 and D2 blockade produced similarly dramatic behavioral effects, it is possible that either increased entrainment or decreased firing rate alone can result in a hypokinetic phenotype, with stronger effect when both occur simultaneously. The near-total immobility caused by concomitant blockade of D1 and D2 type receptors is consistent with this possibility. Furthermore, since D2-type receptors have higher affinity for dopamine than D1 type receptors (Richfield et al., 1989
), these results suggest that changes in striatal synchrony and rate could emerge at different timepoints during Parkinson’s disease progression. D1-mediated effects could emerge earlier in Parkinson’s disease given that small changes in DA levels could affect preferentially the activation of low affinity versus high affinity receptors, while D2-mediated effects could become more prominent as the disease progresses.
In interpreting these results, we should consider that the systemic blockade of D1 and D2 type receptors can cause very complex interactions that stem from places other than the basal ganglia. However, idiopathic PD is in most cases a systemic and widespread DA depletion, and not simply hypodopaminergism of the striatum (Gerlach et al., 1991
; Biehlmaier et al., 2007
). Moreover, as SNc neurons are known to project to the GPe and STN as well as to the striatum, and these structures can modulate striatal activity (Bevan et al., 2002
), localized DA-blockade may not reflect all the interactions resulting from DA depletion in PD, although local manipulations would be better to mechanistically isolate the effects observed.
We should also consider that we used doses of D1 and D2 type receptor antagonists that completely block the receptors, as they block the effects of L-DOPA in restoring movement in DA-depleted animals (Costa et al., 2006
). Therefore, it is possible that the singularity of the results obtained from the complete blockade of only one dopamine receptor type but not the other could arise from the treatment being rather different than dopamine depletion (which typically affects both receptor types) and therefore from an imbalance or a competition between the different receptors and/or pathways (Taverna et al., 2008
), which may never happen to this extreme in a more natural situation. Nonetheless, the fact that across all treatments the probability of a neuron changing firing rate was not related to the probability of changing entrainment to the LFP still indicates that mechanistically, changes in firing rate and entrainment to the local field potential in the dorsal striatum after DA depletion can be somewhat independent. Further, these results suggest that given the complexity of the anatomical and functional interaction between different “nuclei” and different cell types in the basal ganglia (Lévesque and Parent, 2005
; Mallet et al., 2006
; Nadjar et al., 2006
; Taverna et al., 2008
), movement problems arising from DA depletion in Parkinson’s disease are probably overly simplisticly conceptualized.
It is possible that the preferential effects seen after D1- and D2-receptor blockade in MSNs are mediated by striatal interneurons, either GABAergic FSIs or cholinergic LANs, or both. We recorded a small sample of these neurons; however, we were unable to record enough to perform robust statistical analyses. Future investigations should examine the possibility that DA modulation of striatal interneurons affects the firing rate and/or synchrony of striatal MSNs. Although it is very difficult to test the involvement of striatal interneurons using extracellular recordings and global pharmacological manipulations, they may be better investigated using optogenetics or selective elimination.
In summary, the data presented here suggest that although dopamine depletion and dopamine receptor blockade cause both alterations in the firing rate and in the entrainment to the local field potential of medium spiny neurons in the dorsal striatum, these effects seem to arise via different mechanisms, as they emerge preferentially after the blockade of D1- or D2-type receptors, and changes in one do not seem to be related to changes in the other. These results indicate that changes in firing rate and synchrony of striatal neurons may be the result of dissociable and interactive actions of dopamine in the activity of neuronal ensembles in the dorsal striatum.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by the Division of Intramural Clinical and Basic Research of the NIAAA, NIH and the Champalimaud Neuroscience Programme at Instituto Gulbenkian de Ciência.
Burbaud, P., Gross, C., Benazzouz, A., Coussemacq, M., and Bioulac, B. (1995). Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Exp. Brain Res. 105, 48–58.
Marcellino, D., Ferre, S., Casado, V., Cortes, A., Le Foll, B., Mazzola, C., Drago, F., Saur, O., Stark, H., Soriano, A., Barnes, C., Goldberg, S. R., Lluis, C., Fuxe, K., and Franco, R. (2008). Identification of dopamine D1–D3 receptor heteromers. Indications for a role of synergistic D1–D3 receptor interactions in the striatum. J. Biol. Chem. 283, 26016–26025.
Nadjar, A., Brotchie, J. M., Guigoni, C., Li, Q., Zhou, S.-B., Wang, G. J., Ravenscroft, P., Georges, F., Crossman, A. R., and Bezard, E. (2006). Phenotype of striatofugal medium spiny neurons in Parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J. Neurosci. 26, 8653–8661.
Rohlfs, A., Nikkhah, G., Rosenthal, C., Rundfeldt, C., Brandis, A., Samii, M., and Loscher, W. (1997). Hemispheric asymmetries in spontaneous firing characteristics of substantia nigra pars reticulata neurons following a unilateral 6-hydroxydopamine lesion of the rat nigrostriatal pathway. Brain Res. 761, 352–356.