- 1Hearing Sciences, Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, United Kingdom
- 2NIHR Nottingham Biomedical Research Centre, University of Nottingham, Nottingham, United Kingdom
- 3Department of Otorhinolaryngology, Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom
- 4Department of Otorhinolaryngology Head and Neck Surgery, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
At this time of the COVID-19 pandemic, potentially effective treatments are currently under urgent investigation. Benefits of chloroquine and hydroxychloroquine for the treatment of COVID-19 infection have been proposed and clinical trials are underway. Chloroquine and hydroxychloroquine, typically used for the treatment of malaria and autoimmune diseases, have been considered for off-label use in several countries. In the literature, there are reports of ototoxic effects of the drugs causing damage to the inner ear structures, which then result in hearing loss, tinnitus, and/or imbalance. This mini-review represents a summary of the findings from a systematic search regarding ototoxicity of chloroquine and hydroxychloroquine in the published literature. The characteristics of sensorineural hearing loss and/or tinnitus after chloroquine or hydroxychloroquine treatment can be temporary but reports of persistent auditory and vestibular dysfunction exist. These are not frequent, but the impact can be substantial. Additionally, abnormal cochleovestibular development in the newborn was also reported after chloroquine treatment in pregnant women. The suggested dose of chloroquine for COVID-19 infection is considerably higher than the usual dosage for malaria treatment; therefore, it is plausible that the ototoxic effects will be greater. There are potential implications from this review for survivors of COVID-19 treated with chloroquine or hydroxychloroquine. Patient reports of hearing loss, tinnitus, or imbalance should be noted. Those with troublesome hearing loss, tinnitus and/or imbalance are encouraged to be referred for hearing evaluation and interventions once they are stable. Clinical trials of chloroquine or hydroxychloroquine should also consider including audiological monitoring in the protocol.
Introduction
At this time of the COVID-19 global pandemic, potentially effective treatments are currently under urgent investigation. Currently, there is no evidence from randomized clinical trials that any specific therapy improves outcomes in patients with COVID-19 (1). Chloroquine and hydroxychloroquine are considered to be promising repurposed drugs against COVID-19, based on pathophysiological considerations and in vitro results (2, 3). These drugs have received particular attention as they are widely available and inexpensive. Chloroquine and hydroxychloroquine, quinine-related compounds, have been used for the treatment of malaria and chronic inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis. The anti-viral and anti-inflammatory properties may account for the efficacy in treating patients with COVID-19 infection (4). There have been reports that patients who received chloroquine or hydroxychloroquine had faster virological clearance (5, 6), however there are some limitations of the studies such as small sample size and questionable methodology. There is no high-quality evidence of potential benefit of these drugs at the moment. Presently, there are over 80 registered ongoing trials worldwide examining the role of chloroquine and hydroxychloroquine in COVID-19 treatment (7).
Clinical practice guidelines have considered chloroquine and hydroxychloroquine for off-label and compassionate therapies against moderate to severe cases of COVID-19 in several countries including China, Korea, USA, France, Italy, and Belgium (8). There is currently also a massive global demand for chloroquine and hydroxychloroquine as people around the world are self-medicating after health professionals and politicians have endorsed the drugs. Chloroquine and hydroxychloroquine are also freely available in the UK and other countries without prescription.
Some potential side effects of chloroquine and hydroxychloroquine are cardiac arrhythmias, retinopathy, and muscle weakness (4). The clinical and research literature also contains reports of ototoxic effects after chloroquine and hydroxychloroquine treatment. Ototoxicity refers to drug-related injury causing damage to the inner ear structures, which then result in hearing loss and/or tinnitus (the subjective perception of sound such as ringing, hissing, or buzzing, without an external source), and/or imbalance (9). Permanent hearing loss can adversely affect cognitive health (10) and mental well-being (11). Troublesome tinnitus is associated with insomnia, poor concentration, anxiety and depression (12). The mechanisms of chloroquine associated hearing loss include cochlear outer hair cell dysfunction, and inhibition of post synaptic sodium channel function in cochlear spiral ganglion cells (13). Additionally, some alterations in central auditory function, which may trigger tinnitus, have been observed after quinine administration (13).
This mini-review represents a summary of the findings from a literature search regarding ototoxicity of the drugs in the published literature as well as the discussion of potential implications for survivors of COVID-19 so treated.
Method
A systematic literature search on Medline and EMBASE platforms was undertaken on 26th March 2020, updated on 23rd April 2020. The search strategy combined MeSH terms and keywords of chloroquine or hydroxychloroquine, ototoxicity, hearing loss, hearing, tinnitus. English language publications containing relevant data to this review were included. Data extraction items included year, study design, sample size, and audiological outcomes. Data were collated in the table and then summarized by narrative synthesis. Recommendations from audiological professional perspectives were then made.
Results
Chloroquine Ototoxicity
Eleven publications, reporting topics associated with ototoxic effects of chloroquine, were identified and are summarized in Table 1. The year of publication ranged from 1954 to 2015. There were 7 case reports, 2 observational studies, 1 case control study, and 1 review article. The sample size of the study participants varied from 1 to 74.
Ten patients (8 adults and 2 children) in 6 publications had either abnormal audiogram or reported hearing loss after chloroquine treatment. Three out of ten cases had temporary sensorineural hearing loss after chloroquine treatment that improved after cessation of the medication (14, 19). A prospective observational study in 2015 concluded that ototoxic effects of chloroquine at regular doses for malaria treatment (1.2 g daily for 3 days) were fully reversible (14). Sensorineural hearing loss after chloroquine in a 6-year old girl was partially reversible after prednisolone administration (19). However, permanent severe sensorineural hearing loss has also been reported in 2 cases (17, 20). Additionally, reversible chloroquine-induced cochlear injury was detectable by brainstem audiometry in 13 out of 70 patients despite normal pure tone audiogram results (18). Tinnitus has also been reported concurrently with persistent hearing loss in 1 case (20). Imbalance was reported in 3 cases (14, 19, 20).
While there was no difference in hearing thresholds between children who were and were not exposed to chloroquine during gestation (16), there were 3 case reports of intrauterine effects of chloroquine associated with abnormal cochleovestibular development in newborns (21, 22).
Hydroxychloroquine Ototoxicity
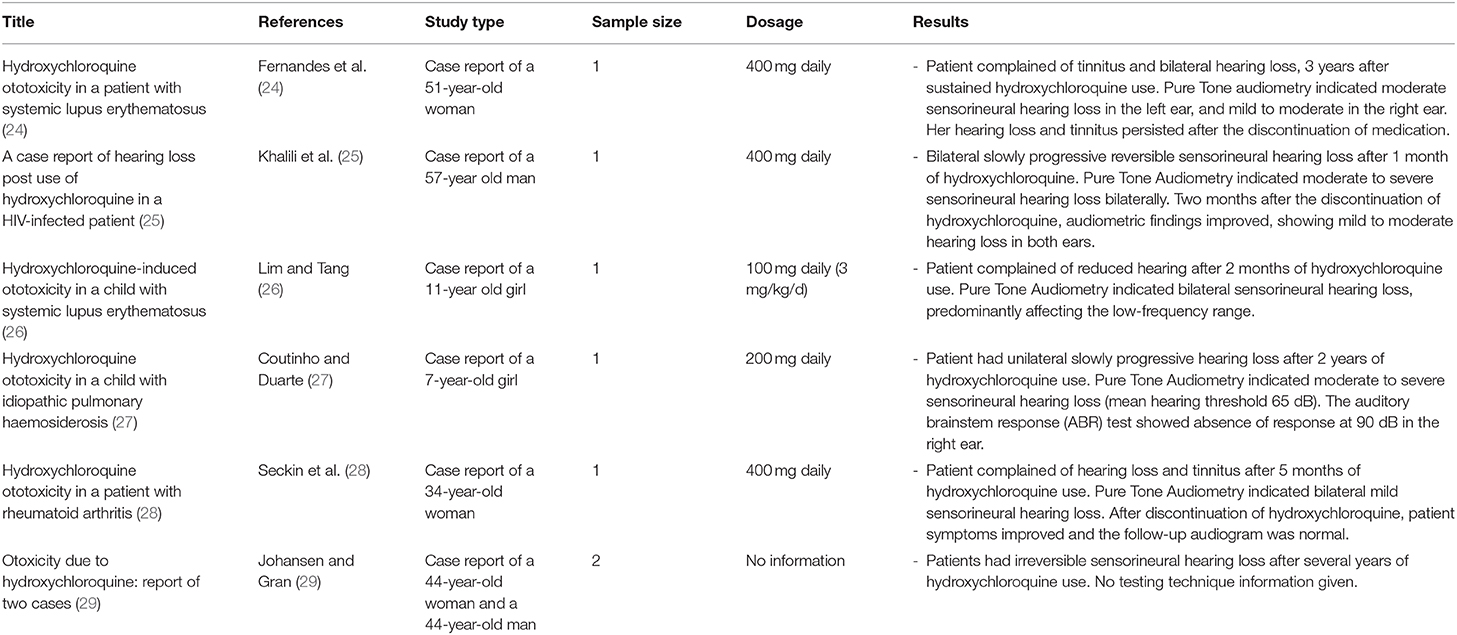
Six case reports, describing ototoxic effects associated with hydroxychloroquine, were identified and are displayed in Table 2. Publication year ranged from 1998 to 2018. Sensorineural hearing loss was identified after hydroxychloroquine treatment in five adults and two children. The sensorineural hearing loss was found to be either reversible (25, 28) or irreversible (24, 29). The onset of hearing loss after hydroxychloroquine treatment varied from 1 month (25) to several years (29). Tinnitus was also reported concomitantly with hearing loss in 2 cases (24, 28).
Discussion
The manifestation of sensorineural hearing loss and/or tinnitus and/or imbalance after chloroquine and hydroxychloroquine can be either temporary or permanent. Most of the studies on this topic were case series or case reports with only a few observational studies. Information from a definitive large study with good methodology is still lacking. Ototoxicity after chloroquine use tends to be more sudden, while hydroxychloroquine is more likely to cause ototoxicity after prolonged use. This could be due to different drug efficacy and equivalent dosage. Furthermore, hearing loss in these patients could be associated with other possible causes rather than chloroquine and hydroxychloroquine including autoimmune disease e.g., systemic lupus erythematosus (30), sudden sensorineural hearing loss or presbycusis.
The suggested dose of chloroquine for patients diagnosed with COVID-19 infection (1 g daily for 10 days) is substantially higher compared with the usual dosage of chloroquine for malaria treatment (1 g daily for 3 days) (5). There is no information regarding the ototoxic effect of chloroquine at this higher dose. Patients with chronic inflammatory diseases were treated with a usual dose of hydroxychloroquine 400 mg daily for long durations (months or years). A suggested dose of hydroxychloroquine for COVID-19 infection is an initial loading dose of 800 mg followed by 400 mg daily for 4 days based on the in vitro model (2), and 600 mg daily for 10 days from a French study (6). In general, the recommended dosage of hydroxychloroquine in COVID-19 patients is slightly higher but in a shorter duration compared to that in autoimmune disease. The ototoxic effects of these regimens are unknown.
Due to the potentially substantial number of the world's population who may take chloroquine or hydroxychloroquine, there is the prospect of a significant number of people being affected with ototoxic side effects. It is therefore vital to build awareness about the presentation and impact of the symptoms of drug-induced ototoxicity. Patient reports of hearing loss, tinnitus, or imbalance should be noted. Those with troublesome hearing loss or tinnitus are encouraged to be referred for hearing evaluation, including extended high frequencies audiometry at 8–16 kHz where possible, once they are stable. Available options of audiological interventions for those with bothersome hearing impairment or tinnitus are counseling, hearing aids, and tinnitus therapy. The possibility of exacerbation of pre-existing hearing loss and/or tinnitus should be considered. Synergistic adverse auditory effects when other ototoxic medication is administered with chloroquine or hydroxychloroquine, such as aminoglycoside antibiotics and azithromycin, is a further risk (9). Severe cases of COVID-19 can also progress to respiratory distress and hypoxia (31). Hypoxia is known to have deleterious effects on the stria vascularis of the cochlea organ including alterations to cochlear potentials and histologic changes (32). Therefore, it is certainly possible that the combined effects of hypoxia and administration of chloroquine or hydroxychloroquine on hearing could be worse than either one alone. Clinical trials of chloroquine or hydroxychloroquine should also consider including audiological monitoring in the protocol. Ideally, a hearing test should be conducted both before and after drug administration to examine drug-induced hearing change. Common methods for audiological evaluation include pure tone audiometry, otoacoustic emission (OAE), and tinnitus questionnaire. However, conventional methods and setting of hearing evaluation is impractical based on the infectious nature of COVID-19 and the urgency of drug administration. Self-monitoring by validated smartphone-based apps for hearing assessments in addition to self-report of symptoms is an approach of interest in this situation.
Although chloroquine and hydroxychloroquine are generally considered safe in pregnant women, the use of chloroquine during pregnancy in the first trimester should be contemplated with particular caution since there are reports of abnormal cochleovestibular development in newborns. Hydroxychloroquine has a safer clinical profile in pregnancy, thus is a more suitable option than chloroquine (33).
Conclusion
Recent publications have brought attention to the possible benefit of chloroquine and hydroxychloroquine in COVID-19 treatment. It is important to build awareness about the possibility of ototoxicity in survivors of COVID-19 treated with these drugs. Patient reports of hearing loss, tinnitus, or imbalance should be noted. Those with troublesome hearing loss or tinnitus are encouraged to be referred for hearing evaluation and interventions once they are stable. Clinical trials of chloroquine or hydroxychloroquine should also consider including audiological monitoring in the protocol.
Author Contributions
All authors planned and structured the paper. PP undertook the review and wrote the first draft. DB, PP, and AK jointly revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PP and DB receive support from the UK National Institute for Health Research (NIHR), but their views are their own and do not reflect those of NIHR nor the UK Department of Health and Social Care.
References
1. Kalil AC. Treating COVID-19-Off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. (2020) 323:1897–8. doi: 10.1001/jama.2020.4742
2. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. (2020). doi: 10.1093/cid/ciaa237. [Epub ahead of print].
3. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
4. Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. (2020) 55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [Epub ahead of print].
5. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–3. doi: 10.5582/bst.2020.01047
6. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrobial Agents. (2020). doi: 10.1016/j.ijantimicag.2020.105949. [Epub ahead of print].
7. Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. (2020) 369:m1432. doi: 10.1136/bmj.m1432
8. Jie Z, He H, Xi H, Zhi Z. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zh. (2020) 43:185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009
9. Cianfrone G, Pentangelo D, Cianfrone F, Mazzei F, Turchetta R, Orlando MP, et al. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. (2011) 15:601–36.
10. Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol. (2017) 2:69–79. doi: 10.1002/lio2.65
11. Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist. (2019) 60:e137–54. doi: 10.1093/geront/gnz009
12. Manchaiah V, Beukes EW, Granberg S, Durisala N, Baguley DM, Allen PM, et al. Problems and life effects experienced by tinnitus research study volunteers: an exploratory study using the ICF classification. J Am Acad Audiol. (2018) 29:936–47. doi: 10.3766/jaaa.17094
13. Baguley D, Fagelson M. Tinnitus: Clinical and Research Perspectives, 1st ed. San Diego, CA: Plural Publishing, Inc (2016).
14. Subramaniam V, Vaswani RV. Assessment of short term chloroquine-induced ototoxicity in malaria patients. Global J Med Res. (2015) 15:14–17.
15. Bortoli R, Santiago M. Chloroquine ototoxicity. Clin Rheumatol. (2007) 26:1809–10. doi: 10.1007/s10067-007-0662-6
16. Borba EF, Turrini-Filho JR, Kuruma KA, Bertola C, Pedalini ME, Lorenzi MC, et al. Chloroquine gestational use in systemic lupus erythematosus: assessing the risk of child ototoxicity by pure tone audiometry. Lupus. (2004) 13:223–7. doi: 10.1191/0961203304lu528oa
17. Hadi U, Nuwayhid N, Hasbini AS. Chloroquine ototoxicity: an idiosyncratic phenomenon. Otolaryngol Head Neck Surg. (1996) 114:491–3. doi: 10.1016/S0194-5998(96)70226-7
18. Bernard P. Alterations of auditory evoked potentials during the course of chloroquine treatment. Acta Otolaryngol. (1985) 99:387–92. doi: 10.3109/00016488509108928
19. Mukherjee DK. Chloroquine ototoxicity–a reversible phenomenon? J Laryngol Otol. (1979) 93:809–15. doi: 10.1017/S0022215100087740
20. Dwivedi GS, Mehra YN. Ototoxicity of chloroquine phosphate. A Case Report J Laryngol Otol. (1978) 92:701–3. doi: 10.1017/S0022215100085960
21. Matz GJ, Naunton RF. Ototoxicity of chloroquine. Arch Otolaryngol. (1968) 88:370–372. doi: 10.1001/archotol.1968.00770010372008
22. Hart CW, Naunton RF. The ototoxicity of chloroquine phosphate. Arch Otolaryngol. (1964) 80:407–412. doi: 10.1001/archotol.1964.00750040419009
23. Dewar WA, Mann HM. Chloroquine in lupus erythematosus. Lancet. (1954) 263:780–1. doi: 10.1016/S0140-6736(54)92747-6
24. Fernandes MRN, Soares DBR, Thien CI, Carneiro S. Hydroxychloroquine ototoxicity in a patient with systemic lupus erythematosus. An Bras Dermatol. (2018) 93:469–70. doi: 10.1590/abd1806-4841.20187615
25. Khalili H, Dastan F, Dehghan Manshadi SA. A case report of hearing loss post use of hydroxychloroquine in a HIV-infected patient. Daru. (2014) 22:20–20. doi: 10.1186/2008-2231-22-20
26. Lim SC, Tang SP. Hydroxychloroquine-induced ototoxicity in a child with systemic lupus erythematosus. Int J Rheum Dis. (2011) 14:e1–2. doi: 10.1111/j.1756-185X.2010.01582.x
27. Coutinho MB, Duarte I. Hydroxychloroquine ototoxicity in a child with idiopathic pulmonary haemosiderosis. Int J Pediatr Otorhinolaryngol. (2002) 62:53–7. doi: 10.1016/S0165-5876(01)00592-4
28. Seckin U, Ozoran K, Ikinciogullari A, Borman P, Bostan EE. Hydroxychloroquine ototoxicity in a patient with rheumatoid arthritis. Rheumatol Int. (2000) 19:203–4. doi: 10.1007/s002960000054
29. Johansen PB, Gran JT. Ototoxicity due to hydroxychloroquine: report of two cases. Clin. Exp. Rheumatol. (1998) 16:472–4.
30. Abbasi M, Yazdi Z, Kazemifar AM, Bakhsh ZZ. Hearing loss in patients with systemic lupus erythematosus. Glob J Health Sci. (2013) 5:102–6. doi: 10.5539/gjhs.v5n5p102
31. Kaul D. An overview of coronaviruses including the SARS-2 coronavirus - Molecular biology, epidemiology and clinical implications. Curr Med Res Pract. (2020). doi: 10.1016/j.cmrp.2020.04.001. [Epub ahead of print].
32. Dziennis S, Reif R, Zhi Z, Nuttall AL, Wang RK. Effects of hypoxia on cochlear blood flow in mice evaluated using Doppler optical microangiography. J Biomed Opt. (2012) 17:106003. doi: 10.1117/1.JBO.17.10.106003
Keywords: chloroquine, hydroxychloroquine, ototoxicity, hearing loss, tinnitus, COVID-19
Citation: Prayuenyong P, Kasbekar AV and Baguley DM (2020) Clinical Implications of Chloroquine and Hydroxychloroquine Ototoxicity for COVID-19 Treatment: A Mini-Review. Front. Public Health 8:252. doi: 10.3389/fpubh.2020.00252
Received: 28 April 2020; Accepted: 20 May 2020;
Published: 29 May 2020.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Kelly M. Reavis, VA Portland Health Care System, United StatesJessica Paken, University of KwaZulu-Natal, South Africa
Copyright © 2020 Prayuenyong, Kasbekar and Baguley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pattarawadee Prayuenyong, msxpp4@nottingham.ac.uk
 Pattarawadee Prayuenyong
Pattarawadee Prayuenyong Anand V. Kasbekar
Anand V. Kasbekar David M. Baguley
David M. Baguley