- 1Department of Botany and Plant Biotechnology, University of Johannesburg, Johannesburg, South Africa
- 2Department of Biochemistry, University of Stellenbosch, Stellenbosch, South Africa
- 3Mass Spectrometry Unit, Central Analytical Facility, University of Stellenbosch, Stellenbosch, South Africa
Stevens’ Cure (Umckaloabo) emerged as a patent medicine claiming to treat tuberculosis in the United Kingdom at the beginning of the 20th century. However, due to its identity being shrouded in secrecy, it was never truly accepted by the medical community. It was “rediscovered” in the 1970s and subsequently developed into a very popular and successful phytopharmaceutical for the treatment of upper respiratory tract infections. Whether Stevens’ Cure contained the same ingredient(s) as the modern Umckaloabo has not yet been demonstrated. We attempted to elucidate for the first time the identity of the original ingredient by comparative analysis of historical product samples. Three historical samples of Stevens’ Cure were compared with Pelargonium sidoides DC. and P. reniforme Curt. root per UPLC-MS analysis. We confirm that the ingredient–P. sidoides DC.—is indeed the same as used in modern phytotherapy. We also attribute the first ethnopharmacological record of P. sidoides DC. being used for the treatment of tuberculosis to C. H. Stevens, the “creator” of Umckaloabo.
1 Introduction
Stevens’ Cure, today better known as Umckaloabo, was introduced to the United Kingdom as a patent medicine at the end of the 19th century. Similar to other early introductions of southern African medicinal herbs, such as devil’s claw (Harpagophytum spp.), rooibos [Aspalathus linearis (Burm.f.) R.Dahlgren], honeybush (Cyclopia spp.), buchu (Agathosma spp.), Cape aloe (Aloe ferox Mill.) and uzara [Xysmalobium undulatum (L.) W.T. Aiton] (Stander et al., 2019; Brendler, 2021; Brendler and Abdel-Tawab, 2022; Brendler and Cock, 2022; Brendler et al., 2023), its arrival in Europe was driven by entrepreneurship and, as opposed to later (and less successful) attempts, such as hoodia [Hoodia gordonii (Masson) Sweet ex Decne] and sceletium (Mesembryanthemum tortuosum L.) (Brendler, 2020; Brendler et al., 2021), uninhibited by regulations. Suffering from tuberculosis, Charles H. Stevens was sent by his doctor for convalescence to South Africa, where he was miraculously cured by a local Sotho healer. He brought the cure, an unidentified botanical, back to England and began to market it to fellow sufferers as a patent medicine branded as Umckaloabo. Aggressive advertising (by mail, in dailies, through anonymously published books and paraphernalia such as bookmarks) made his business flourish. Stevens was open to the medical establishment, promoting his remedy on multiple occasions, however, his secrecy and claims of a cure for tuberculosis did not pair well with the scrutiny of science and he was largely ignored or labelled a quack (British Medical Association, 1909a; British Medical Association, 1909b; American Medical Association, 1910; American Medical Association, 1930). It is Stevens’ contentious nature that we owe the opportunity to–belatedly–uncover his secret. He pressed multiple litigations against the medical establishment (British Medical Association, 1912) but lost due to his unwillingness to disclose the identity of his ingredient, among other reasons. However, every one of those court cases led to attempts to elucidate the composition of his remedy: samples were procured, government officials tasked, and renowned botanical institutes involved (Misc, 1936ff.). Even though none of these attempts were successful, they left a paper trail and the samples ended up in official collections. We were able to locate four samples in the Economic Botany Collection of the Royal Botanical Gardens, Kew, three of which could be authenticated as Stevens’ Cure beyond a reasonable doubt (see Section 2.1). These were analyzed and results are presented below. Stevens’ success also caught international attention, and the Swiss French physician Adrien Sechehaye started to treat his patients with Umckaloabo and reported his successes to the European medical societies (Sechehaye, 1923; Sechehaye, 1934). Soon, interest was raised also in Germany (Bojanowski, 1937). That may be the reason for why after Stevens’ death the “brand” and its secret ended up there. In parallel, however, antibiotics became available for the treatment of tuberculosis, and Umckaloabo fell to the wayside until it was revived by the quest of German scientist Sabine Bladt in the early 1970s (Bladt, 1974). Bladt, curiously, was unaware of the historic samples, or at least nothing in her published work hints at her knowing of their existence. Her ethnobotanical and biochemical approach led to the identification of Pelargonium reniforme as the source plant of Umckaloabo (Bladt, 1977). In retrospect, it must be assumed that her collected plant material was not correctly identified (pers. comm. K. P. Latté, July 2020), since she reported the presence of umckalin, which was subsequently found to be the marker compound for P. sidoides (Kayser, 1997), while absent in P. reniforme (Latté, 1999). In fact, Kolodziej and co-workers (Kolodziej, 2000; Kolodziej et al., 2002; Kolodziej, 2007) have identified several compounds including coumarin and coumarin sulfates (umckalin and umckalin sulfate) in P. sidoides that were not detected in P. reniforme. The presence of umckalin as a unique chemical marker for P. sidoides was also confirmed by Viljoen and colleagues (Viljoen et al., 2015).
The transformation of Umckaloabo from a patent medicine into a modern phytopharmaceutical has been reviewed by us previously (Brendler and van Wyk, 2008; Brendler, 2009). Since then, more than 100 publications have been added to the already impressive body of data on biochemistry, pharmacology, clinical efficacy, and safety of Umckaloabo. The contemporary brand owner (Schwabe Group, Karlsruhe, Germany) has promoted the investigation of Umckaloabo (EPs® 76301) resulting in more than 30 clinical trials over the last 25 years (total study population >10,000) for the treatment of acute respiratory tract infections. A Cochrane review of eight studies (Timmer et al., 2013) found some evidence for efficacy but deemed the overall quality low. More recent reviews (Matthys et al., 2016; Careddu and Pettenazzo, 2018; Seifert et al., 2019) included a larger body of data in their reviews and meta-analyses, and attested efficacy in children, adolescents and adult patients with acute bronchitis, rhinosinusitis or tonsillopharyngitis. An investigation of EPs® 7630 effect on respiratory viruses (Michaelis et al., 2011) and its excellent safety profile (Kamin et al., 2018; Schapowal et al., 2019) led to it being discussed as having potential to affect the human immune response in the context of COVID-19 (Brendler et al., 2020), which has since been confirmed in vitro and in vivo (Papies et al., 2021; Emanuel et al., 2023).
2 Materials and methods
2.1 Samples and sampling
Commercial samples of dried Pelargonium sidoides and P. reniforme roots were supplied by Ulrich Feiter of Parceval (Pty) Ltd., Wellington, South Africa. The batch numbers were 20090, 20091, and 20092 for P. sidoides, and 2023, 20094, and 20095 for P. reniforme. The three historic samples, EBC 45821, EBC 45819 and EBC 77377 were procured from the Economic Botany Collection of the Royal Botanic Gardens, Kew, United Kingdom. The authenticity of those samples could be confirmed with the help of catalogue notes and by relating them to a file labelled “Umckaloaba” [sic!] of various correspondence regarding the British Medical Association’s attempts to identify the active ingredient in Stevens’ Cure (Misc, 1936ff.) (EBS 45821 and EBS 77377), whereas EBS 45819 was contained in an envelope addressed to the Royal Botanic Gardens from Pharmacie Hahn, Geneva, 1922. Adrien Sechehaye mentioned this pharmacy as his supplier for Umckaloabo (Helmstädter, 1996).
2.2 Extraction
Approximately 0.2 g of dry plant material was extracted with 50% methanol in water containing 1% formic acid (2 mL) in a 15 mL polypropylene centrifuge tube by soaking it overnight, followed by extraction in an ultrasonic bath (0.5 Hz, Integral systems, RSA) for 60 min at room temperature. The extracts were centrifuged (Hermle Z160m, 3,000 × g for 5 min) to remove any particulates and transferred into vials. An additional analysis was performed on the hydrolyzed samples.
2.3 Standards
Standards of umckalin, gallic acid and citric acid were obtained from Sigma-Aldrich. Standard solutions of umckalin were prepared quantitatively ranging from 1 to 100 μg/mL in concentration in methanol.
2.4 LC-MS analysis
A Waters Synapt G2 Quadrupole time-of-flight (QTOF) mass spectrometer (MS) connected to a Waters Acquity ultra performance liquid chromatograph (UPLC) (Waters, Milford, MA, United States) was used for high resolution UPLC-MS analysis. In short electrospray ionization in the negative mode was used, a Waters HSS T3, 2.1 mm × 100 mm, 1.7 μm column and mobile phase gradient of 0.1% formic acid (Solvent A) and acetonitrile containing 0.1% formic acid as solvent B (Stander et al., 2017). The flow rate was 0.25 mL/min, the gradient started with a 1-min hold at 100% (A) followed by a linear gradient to 28% solvent (B) in 22 min and another linear gradient over 50 s to 40%, a wash step at 100% (B) for a minute and re-equilibration to initial conditions over 4 min to give a total run time of 29 min. The column was at 60°C. Data was acquired in MSE mode where both low energy data and high energy fragmentation data are acquired.
3 Results
Direct evidence of the botanical identity of the three historical samples of Stevens’ Cure is revealed here for the first time (Figure 1).
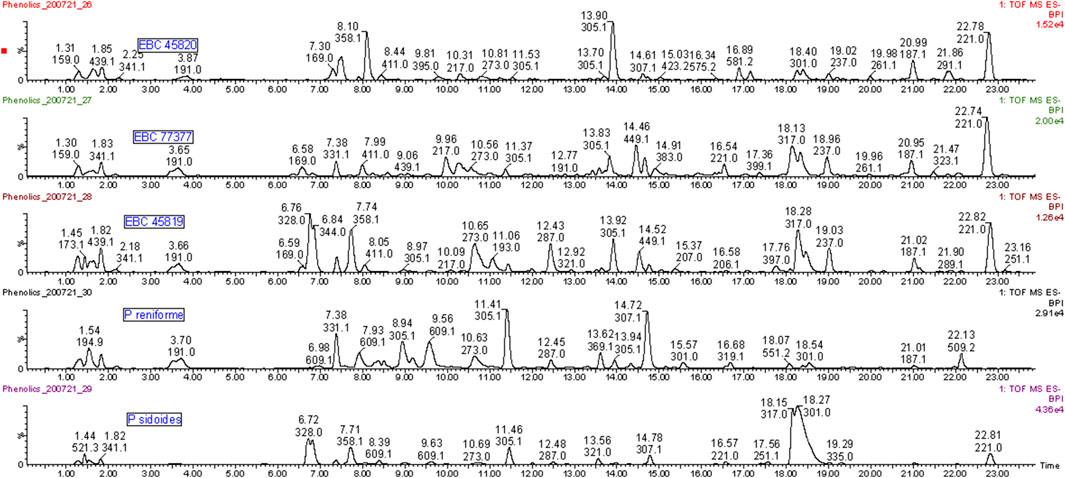
FIGURE 1. Phenolic compounds in extracts from authentic root samples of Pelargonium reniforme, P. sidoides and three historical samples of Stevens’ Cure (EBC 45819, EBC 77377, EBC 45820). Umckalin (Rt ∼22.8 min) is a known marker compound for P. sidoides. Umckalin sulfate (Rt ∼18.3 min) occurs at high levels in P. sidoides and the Kew samples but not in P. reniforme. Epigallocatechin (Rt 11.4 min) and gallocatechin (Rt 8.9 min) are major compounds in P. reniforme and only minor compounds in P. sidoides and the Kew samples. The turgorins (m/z 328 and 358, 6.7 and 8.09 min) are present in the historical samples and P. sidoides and not in P. reniforme.
Umckalin (m/z 221.0454, 22.8 min) is a known chemical marker for P. sidoides (Viljoen et al., 2015) and occurred as a major phenolic compound in all three of the Kew samples (363–738 mg/kg in the latter–see Figure 1; Supplementary Material).
Umckalin sulfate (6-hydroxy-5,7-dimethylcoumarin-8-sulfate, m/z 301.0009, 17.45 min), is a major compound in P. sidoides (Kolodziej, 2000; Kolodziej et al., 2002; Kolodziej, 2007) and in two of the three museum samples and a minor compound in the third. Epigallocatechin (m/z 305.0664, 11.1 min) is a major compound in P. reniforme and only a minor one in P. sidoides and the three historical samples. Note, that there are more than 1 m/z 305 peaks in Figure 1—the one at 11.4 min is epigallocatechin, the one at 8.9 min (P. reniforme only) is gallocatechin and the one at 13 min an unidentified flavonoid sulfate. Sulfated flavonoids are an uncommon group of compounds but have been found in some plant families. Teles et al. (2018) recently published a review on these compounds. Persicarin (isorhamnetin-3-sulfate) is the first sulfated flavonoid reported, the sulfate is an O-sulfate, whereas with umckalin-sulfate it is a C-sulfate. O-sulfates forms a strong [HSO4−] m/z 96.96 fragment, whereas C-sulfates forms a [M-H-SO3]− as a main fragment ion. See Figure 2 for the structures and Supplementary Material for the spectra and fragmentation information of the marker compounds. An overview of compounds tentatively identified in Pelargonium extracts in this study is provided in Table 1.

FIGURE 2. Structure of the main markers of Pelargonium sidoides (umckalin, umckalin sulfate, gallocatechin and epi-gallocatechin) and persicarin (isorhamnetin-3-sulfate), the first isolated sulfated flavonoid (left to right).
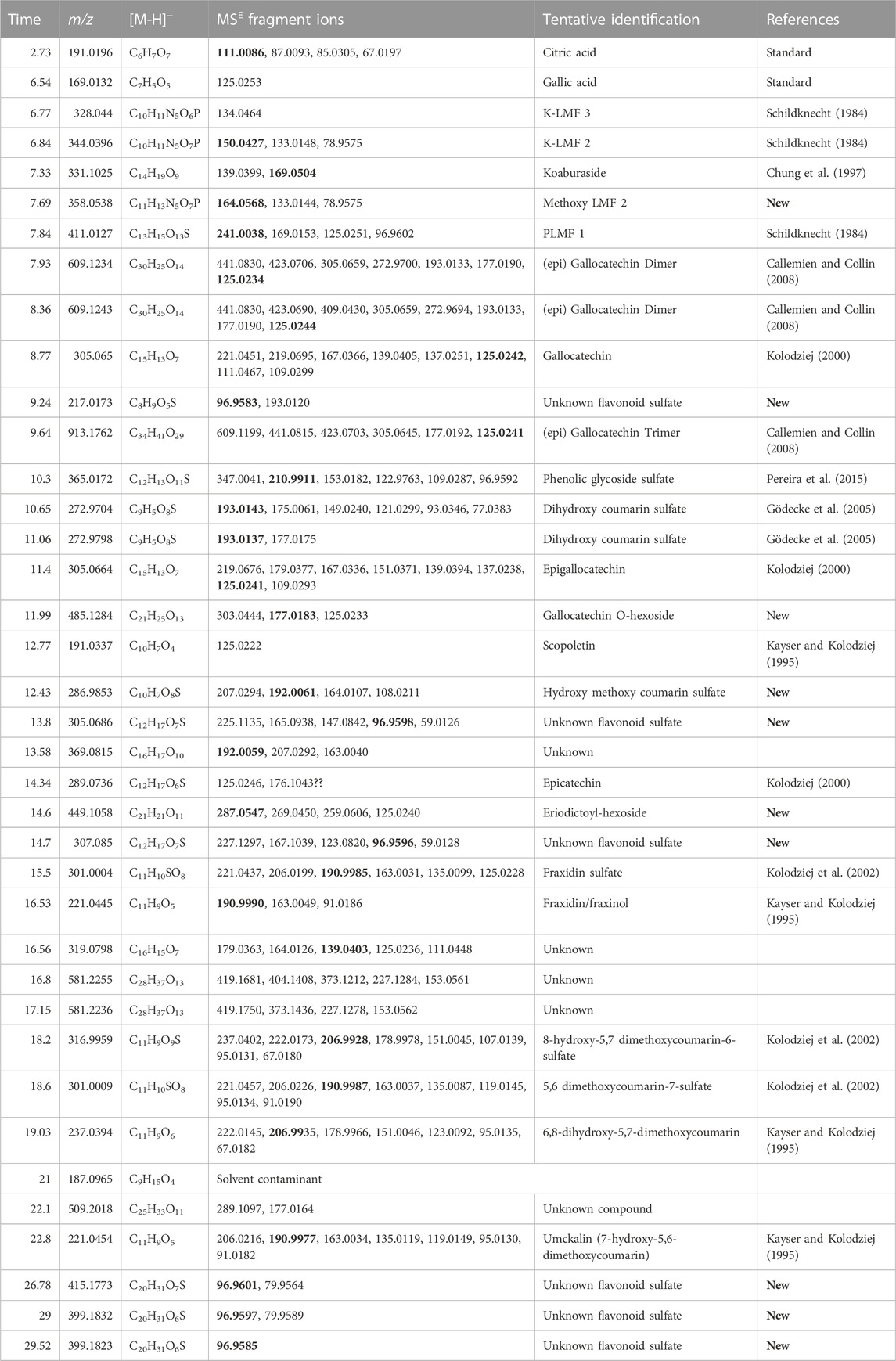
TABLE 1. List of compounds tentatively identified in Pelargonium extracts in this study showing, retention time, detected [M-H] ion, elemental composition and MSE fragments (with the base peaks in bold) as well as literature references to where the compounds were previously detected.
The coumarin sulfates can be distinguished from the flavonoid sulfates in the fragment ions. The coumarin sulfates have a base peak or strong fragment ion at [M-H-SO3]− where the sulfate is on the coumarin ring whereas if the sulfate is on a flavonoid glycoside on another position, a fragment ion at m/z 96.96 is detected for [HSO4]−. It is challenging to identify the sulfated flavonoid glycosides, because only the elemental composition and the one fragment (m/z 96.96) is mostly detected and the compounds are not stable enough to isolate preparatively (Schötz, K., Nöldner, 2006).
Interestingly, sulfated flavonoids have been shown to have antimicrobial affects and they are also some of the main metabolites found in human blood after administration of the aglycones (Teles et al., 2018).
The turgorins (LMF 3, m/z 328.0440, 6.77; LMF 2, m/z 344.0596, 6.8 min) are present in the historical samples and P. sidoides but not in P. reniforme (Schildknecht, 1984).
4 Discussion
While the presence of a small amount of P. reniforme in the historical samples cannot be ruled out with certainty, it is safe to state that most of the plant material is from P. sidoides. The quantitative results are presented in the Supplementary Material. In early attempts to elucidate the composition of Stevens’ Cure it was speculated that it may belong to the genus Rumex (catalogue note to EBS 45819). However, no unique peaks corresponding to the presence of anthraquinones (chrysophanol and its glycosides) were detected in the samples that were not present in P. sidoides in both positive (data not shown) and negative mode.
Tuberculosis and/or respiratory ailments do not appear in the historical southern African literature prior to the 20th century (Brendler and van Wyk, 2008). The earliest record by Sanderson (ca. 1860) is from the Eastern Free State (adjoining Lesotho), where Khoi people were said to use the plant as a cure for unspecified ailments (Smith, 1966).
The earliest record from the Eastern Cape documented the species as iYeza lezikhali and iKhubalo in isiXhosa and reported it to be used for “dysentery, attended by inflammation and fever” (Smith, 1895). In Lesotho, P. sidoides was known as khoaara e nyenyane (in Sesotho) and the roots used to treat colic (Phillips, 1917). Stevens claimed that the plant was known as Umckaloabo in Basutoland (now Lesotho) and that the treatment prescribed by a Basotho healer completely cured him of his tuberculosis (Sechehaye, 1934). We now know that the given provenance of the plant, which included the Gold Coast and Liberia (Anonymous, 1931), and the botanical affinity given as Polygonaceae, were deliberate attempts at hiding the geographical origin and identity of the plant material. Nevertheless, the recorded use against tuberculosis is a valuable original ethnobotanical record for the species, that may have been completely forgotten had it not been for Stevens.
Pelargonium sidoides occurs neither in the Western Cape Province, nor in KwaZulu-Natal Province and Eswatini. The report by Watt and Breyer-Brandwijk (Watt and Breyer-Brandwijk, 1962) that the plant was used in the Cape as an “old remedy for delay in the onset of menses” was based on Kling (Kling, 1923), who merely cited the well-established history of this indication for P. grossularioides (L.) L’Herit (at the time known as P. anceps DC.). The confusion was due to the shared Malay/Afrikaans vernacular names (rabas or rabassam), first recorded by Pappe (Pappe, 1847; 1850) and later by Kling (1923) and Laidler (Laidler, 1928). Therefore, data in Watt and Breyer-Brandwijk (1962) were probably inaccurate but taken at face value by later authors (Hutchings et al., 1996). These authors added the treatment of severe diarrhea, a prolapsed rectum, severe gonorrhea, and a stomach ailment in babies known as intisila to the repertoire of the species, claimed to be used by the Zulu and Swazi.
5 Conclusion
The analysis of historical samples leaves no doubt that Stevens’ Cure was prepared from Pelargonium sidoides, a Sotho medicine traditionally used in Lesotho against colic and, according to Stevens (Sechehaye, 1934), also against tuberculosis. Recorded uses in South Africa do not include respiratory ailments until the late 20th century. Although Stevens’ claim of a cure for tuberculosis was never validated beyond pre-clinical investigations (Seidel and Taylor, 2004; Kim et al., 2009; Qasaymeh et al., 2019), the use of his remedy is vindicated, not only by its botanical identity but also by several clinical studies showing support for the treatment of respiratory infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
TB: Conceptualization, Investigation, Project administration, Resources, Writing–original draft, Writing–review and editing. MS: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. B-EW: Conceptualization, Investigation, Resources, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial support from the National Research Foundation of South Africa (to the National Research Chair in Indigenous Plant Use, NRF Grant Number 84442) and the University of Johannesburg is gratefully acknowledged.
Acknowledgments
We wish to thank the following institutions and persons and acknowledge their contributions: Royal Botanic Garden, Kew, United Kingdom, specifically Prof. Monique Simmonds OBE and Dr. Mark Nesbitt; Mr. Ulrich Feiter of Parceval (Pty) Ltd., Wellington, South Africa for access to the collections and samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fphar.2023.1294997/full#supplementary-material
Footnotes
1Registered trademark of Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany
References
American Medical Association (1910). Exposure of a quack. JAMA 55, 872. doi:10.1001/jama.1910.04330100061021
American Medical Association (1930). Stevens' consumption cure. Declared a fraud and debarred from the mails. JAMA 95, 951. doi:10.1001/jama.1930.02720130047027
Anonymous (1931). Tuberculosis - its treatment and cure with the help of Umckaloabo. London: B. Fraser & Co.
Bladt, S. (1974). Zur Chemie der Inhaltsstoffe der Pelargonium reniforme Curt. - wurzel (Umckaloabo). PhD. Munich, Germany: Ludwig-Maximilians-Universität.
Bladt, S. (1977). Umckaloabo - droge der afrikanischen Volksmedizin. Dtsch. Apoth. Ztg. 117, 1655–1660.
Brendler, T. (2021). From bush medicine to modern phytopharmaceutical: a bibliographic review of devil’s claw (harpagophytum spp). Pharmaceuticals 14, 726. doi:10.3390/ph14080726
Brendler, T. (2009). “Umckaloabo: from a patent remedy to a modern herbal pharmaceutical based on Pelargonium sidoides with clinically proven efficacy,” in African natural plant products I: new discoveries and challenges in chemistry and quality, ACS symposium series. Editor R. Julianiet al. (Washington, DC: ACS Publications), 295–319.
Brendler, T. (2020). “The rise and fall of hoodia: a lesson on the art and science of natural product commercialization,” in African natural plant products III: discoveries and innovations in chemistry, bioactivity, and applications, ACS symposium series. Editor R. Julianiet al. (Washington, DC: ACS Publications), 313–324.
Brendler, T., and Van Wyk, B.-E. (2008). A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 119, 420–433. doi:10.1016/j.jep.2008.07.037
Brendler, T., and Abdel-Tawab, M. (2022). Buchu (Agathosma betulina and A. crenulata): rightfully forgotten or underutilized? Front. Pharmacol. 13, 813142. doi:10.3389/fphar.2022.813142
Brendler, T., Al-Harrasi, A., Bauer, R., Gafner, S., Hardy, M. L., Heinrich, M., et al. (2020). Botanical drugs and supplements affecting the immune response in the time of COVID-19: implications for research and clinical practice. Phytother. Res. 35, 3013–3031. doi:10.1002/ptr.7008
Brendler, T., Brinckmann, J. A., Feiter, U., Gericke, N., Lang, L., Pozharitskaya, O. N., et al. (2021). Sceletium for managing anxiety, depression and cognitive impairment: a traditional herbal medicine in modern-day regulatory systems. Curr. Neuropharm. 19, 1384–1400. doi:10.2174/1570159X19666210215124737
Brendler, T., Cameron, S., and Kuchta, K. (2023). Uzara (Xysmalobium undulatum) - an underutilized anti-diarrhoeic and spasmolytic herbal remedy. J. Ethnopharmacol. 318, 116999. doi:10.1016/j.jep.2023.116999
Brendler, T., and Cock, I. E. (2022). Cape aloe bitters – past and present. S. Afr. J. Bot. 147, 1016–1026. doi:10.1016/j.sajb.2021.11.054
British Medical Association (1909a). Secret remedies: what they cost and what they contain. London: British Medical Association.
British Medical Association (1912). Stevens v. British medical association. BMJ 1250–1255, 1341–1344. 1170–1171.
Callemien, D., and Collin, S. (2008). Use of RP-HPLC-ESI (–)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J. Am. Soc. Brew. Chem. 66, 109–115. doi:10.1094/asbcj-2008-0215-01
Careddu, D., and Pettenazzo, A. (2018). Pelargonium sidoides extract EPs 7630: a review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int. J. Gen. Med. 11, 91–98. doi:10.2147/IJGM.S154198
Chung, M.-I., Lai, M.-H., Yen, M.-H., Wu, R.-R., and Lin, C.-N. (1997). Phenolics from Hypericum geminiflorum. Phytochemistry 44, 943–947. doi:10.1016/s0031-9422(96)00644-9
Emanuel, J., Papies, J., Galander, C., Adler, J. M., Heinemann, N., Eschke, K., et al. (2023). In vitro and in vivo effects of Pelargonium sidoides DC. root extract EPs® 7630 and selected constituents against SARS-CoV-2 B.1, Delta AY.4/AY.117 and Omicron BA.2. Front. Pharmacol. 14, 1214351. in press. doi:10.3389/fphar.2023.1214351
Gödecke, T., Kaloga, M., and Kolodziej, H. (2005). A phenol glucoside, uncommon coumarins and flavonoides from Pelargonium sidoides DC. Z. Naturforsch. B 60, 677–682. doi:10.1515/znb-2005-0612
Hutchings, A., Scott, A. H., Lewis, G., and Cunningham, A. (1996). Zulu medicinal plants. Pietermaritzburg: Natal University Press.
Kamin, W., Funk, P., Seifert, G., Zimmermann, A., and Lehmacher, W. (2018). EPs 7630 is effective and safe in children under 6 years with acute respiratory tract infections: clinical studies revisited. Curr. Med. Res. Opin. 34, 475–485. doi:10.1080/03007995.2017.1402754
Kayser, O. (1997). Phenolische Inhaltsstoffe von Pelargonium sidoides DC. und Untersuchungen zur Wirksamkeit der Umcka-Droge (Pelargonium sidoides DC. und Pelargonium reniforme Curt.). Berlin: PhD Freie Universität.
Kayser, O., and Kolodziej, H. (1995). Highly oxygenated coumarins from Pelargonium sidoides. Phytochemistry 39, 1181–1185. doi:10.1016/0031-9422(95)00166-5
Kim, C. E., Griffiths, W. J., and Taylor, P. W. (2009). Components derived from Pelargonium stimulate macrophage killing of Mycobacterium species. J. Appl. Microbiol. 106, 1184–1193. doi:10.1111/j.1365-2672.2008.04085.x
Kolodziej, H. (2000). Traditionally used Pelargonium species: chemistry and biological activity of umckaloabo extracts and their constituents. Curr. Top. Phytochem. 3, 77–93.
Kolodziej, H. (2007). Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo. Phytomedicine 14, 9–17. Suppl 6. doi:10.1016/j.phymed.2006.11.021
Kolodziej, H., Kayser, O., and Tan, N. (2002). “Novel coumarin sulphates from Pelargonium sidoides: isolation, structure and synthetic approach,” in Natural products in the new millennium: prospects and industrial application. Editors A. Rauter, E. Arauja, F. Sales, J. Justino, and S. P. Santos (Dordrecht: Springer), 59–64.
Latté, K. P. (1999). Phytochemische und pharmakologische Untersuchungen an Pelargonium reniforme Curt. PhD. Berlin: Freie Universität.
Matthys, H., Lehmacher, W., Zimmermann, A., Brandes, J., and Kamin, W. (2016). EPs 7630 in acute respiratory tract infections–a systematic review and meta-analysis of randomized clinical trials. J. Lung. Pulm. Respir. Res. 3, 00068. doi:10.15406/jlprr.2015.03.00068
Michaelis, M., Doerr, H. W., and Cinatl, J. (2011). Investigation of the influence of EPs(R) 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 18, 384–386. doi:10.1016/j.phymed.2010.09.008
Papies, J., Emanuel, J., Heinemann, N., Kulić, Ž., Schroeder, S., Tenner, B., et al. (2021). Antiviral and immunomodulatory effects of Pelargonium sidoides DC. root extract EPs® 7630 in SARS-CoV-2-infected human lung cells. Front. Pharmacol. 12, 757666. doi:10.3389/fphar.2021.757666
Pappe, L. (1847). A list South African indigenous plants used as remedies by the colonists of the Cape of Good Hope. Cape Town: Pike.
Pereira, A., Bester, M., Soundy, P., and Apostolides, Z. (2015). Activity-guided isolation and identification of the major antioxidant and anticancer compounds from a commercial Pelargonium sidoides tincture. Med. Chem. Res. 24, 3838–3852. doi:10.1007/s00044-015-1425-6
Phillips, E. P. (1917). A contribution to the flora of the Leribe Plateau and environs. Ann. S. Afr. Mus. 16, 1–379.
Qasaymeh, R. M., Rotondo, D., Oosthuizen, C. B., Lall, N., and Seidel, V. (2019). Predictive binding affinity of plant-derived natural products towards the protein kinase G enzyme of Mycobacterium tuberculosis (Mt PknG). Plants 8, 477. doi:10.3390/plants8110477
Schapowal, A., Dobos, G., Cramer, H., Ong, K. C., Adler, M., Zimmermann, A., et al. (2019). Treatment of signs and symptoms of the common cold using EPs 7630 - results of a meta-analysis. Heliyon 5, e02904. doi:10.1016/j.heliyon.2019.e02904
Schildknecht, H. (1984). Turgorins—new chemical messengers for plant behaviour. Endeavour 8, 113–117. doi:10.1016/0160-9327(84)90003-6
Sechehaye, A. (1923). Nouvelle méthode de traitment des diverses formes de la tuberculose par l'umckaloabo. Rev. Med. Suisse Romande 43, 185–192.
Sechehaye, A. (1934) The treatment of pulmonary and surgical tuberculosis with Umckaloabo. Internal medication (Stevens' cure). London: Fraser & Co.
Seidel, V., and Taylor, P. W. (2004). In vitro activity of extracts and constituents of Pelagonium against rapidly growing mycobacteria. Int. J. Antimicrob. Agents 23, 613–619. doi:10.1016/j.ijantimicag.2003.11.008
Seifert, G., Brandes-Schramm, J., Zimmermann, A., Lehmacher, W., and Kamin, W. (2019). Faster recovery and reduced paracetamol use - a meta-analysis of EPs 7630 in children with acute respiratory tract infections. BMC Pediatr. 19, 119. doi:10.1186/s12887-019-1473-z
Smith, A. (1895). A contribution to the South African materia medica. 3. Cape Town: J. C. Juta & Co.
Smith, C. A. (1966). Common names of South African plants. Pretoria: Department of Agricultural Technical Services.
Stander, M. A., Brendler, T., Redelinghuys, H., and Van Wyk, B. E. (2019). The commercial history of Cape herbal teas and the analysis of phenolic compounds in historic teas from a depository of 1933. J. Food Compos. Anal. 76, 66–73. doi:10.1016/j.jfca.2018.11.001
Stander, M. A., Van Wyk, B.-E., Taylor, M. J. C., and Long, H. S. (2017). Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J. Agric. Food Chem. 65, 10270–10281. doi:10.1021/acs.jafc.7b03942
Teles, Y. C. F., Souza, M. S. R., and Souza, M. F. V. (2018). Sulphated flavonoids: biosynthesis, structures, and biological activities. Molecules 23, 480. doi:10.3390/molecules23020480
Timmer, A., Gunther, J., Motschall, E., Rucker, G., Antes, G., and Kern, W. V. (2013). Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database Syst. Rev. CD006323. doi:10.1002/14651858.CD006323.pub3
Viljoen, A. M., Zhao, J., Sandasi, M., Chen, W., and Khan, I. A. (2015). Phytochemical distinction between Pelargonium sidoides (“Umckaloabo”) and P. reniforme through 1H-NMR and UHPLC–MS metabolomic profiling. Metabolomics 11, 594–602. doi:10.1007/s11306-014-0722-2
Keywords: Umckaloabo, Stevens’ Cure, Pelargonium sidoides DC., Pelargonium reniforme Curt., umckalin, identification
Citation: Brendler T, Stander MA and van Wyk B-E (2024) Stevens’ Cure (Umckaloabo)—the vindication of a patent medicine. Front. Pharmacol. 14:1294997. doi: 10.3389/fphar.2023.1294997
Received: 15 September 2023; Accepted: 08 December 2023;
Published: 03 January 2024.
Edited by:
David Katerere, Tshwane University of Technology, South AfricaReviewed by:
Cloudius Sagandira, Council for Scientific and Industrial Research (CSIR), South AfricaVeronique Seidel, University of Strathclyde, United Kingdom
Copyright © 2024 Brendler, Stander and van Wyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. Brendler, dHhiQHBsYW50YXBoaWxlLmV1
 T. Brendler
T. Brendler M. A. Stander
M. A. Stander B.-E. van Wyk1
B.-E. van Wyk1