- 1National Centre for Naturopathic Medicine, Southern Cross University, Lismore, NSW, Australia
- 2Office of Research, Endeavour College of Natural Health, Brisbane, QLD, Australia
- 3Australian Research Centre in Complementary and Integrative Medicine, University of Technology, Sydney, NSW, Australia
- 4National Institute of Complementary Medicine (NICM) Health Research Institute, Western Sydney University, Sydney, NSW, Australia
- 5Supportive Care, Chris O’Brien Lifehouse Cancer Hospital, Sydney, NSW, Australia
- 6Clinical School of Medicine, University of Sydney, Sydney, NSW, Australia
- 7Prince of Wales Private Hospital, Centre for Minimally Invasive Neurosurgery, Sydney, NSW, Australia
Background: Cannabis for cancer is very topical and, given the use of illicit cannabis preparations used in this vulnerable population, research investigating standardised, quality-assured medicinal cannabis is critical to inform clinicians and assist patient safety.
Methods: A randomized trial involving adult patients diagnosed with a high-grade glioma, no history of substance abuse, liver or kidney damage or myocardial infarction were eligible for inclusion in a tolerability study on two different ratios of medicinal cannabis. Baseline screening of brain morphology, blood pathology, functional status, and cognition was conducted. A retrospective control group was used for comparison for secondary outcomes.
Results: Participants (n=88) were on average 53.3 years old. A paired t-test assessed the Functional Assessment of Cancer Therapy for Brain Cancer (FACT-Br) between groups from baseline to week 12 found that the 1:1 ratio favoured both physical (p=0.025) and functional (p=0.014) capacity and improved sleep (p=0.009). Analysis of changes from baseline to week 12 also found 11% of 61 participants had a reduction in disease, 34% were stable, 16% had slight enhancement, and 10% had progressive disease. No serious adverse events occurred. Side effects included dry mouth, tiredness at night, dizziness, drowsiness.
Conclusion: This study demonstrated that a single nightly dose of THC-containing medicinal cannabis was safe, had no serious adverse effects and was well tolerated in patients. Medicinal cannabis significantly improved sleep, functional wellbeing, and quality of life.
Clinical Trial Registration: Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373556&isReview=true, identifier ACTRN12617001287325.
Introduction
In recent years, the medicinal properties of the Cannabis genus have received increased attention from researchers around the world alongside regulatory changes in many jurisdictions affecting clinician and public access to cannabis. Current evidence suggests medicinal cannabis (MC) – defined here as cannabis being used for medical purposes - may inhibit chemotherapy-induced nausea and vomiting, stimulate appetite, reduce pain, and decrease inflammation and cancer cell proliferation and survival (1, 2). Based on these effects, numerous medical conditions and health complaints have been identified to potentially benefit from the clinical application of cannabis. These include cancer-related refractory nausea, anorexia and pain, chronic pain conditions, sleep disturbances, refractory epilepsy, multiple sclerosis, muscle spasms and seizures, with studies continuing in Parkinson’s disease, hypoxic brain injury, movement disorders, Alzheimer’s disease among others (2–4).
The pharmacological properties of cannabis are primarily linked to the activity of cannabinoids, a group of terpenophenolic constituents (5). Currently, over 140 cannabinoids have been identified (6, 7). The compound which has been most widely studied is delta-9– tetrahydrocannabinol (THC) while the other main cannabinoid compound with reported beneficial properties is cannabidiol (CBD) (8). THC has diverse therapeutic actions (9–23). It is a partial agonist of cannabinoid receptor type 1 and 2 (CB1 and CB2) (24, 25), expressing similarity to the endogenous cannabinoid anandamide (23, 26–28). THC is the main intoxicating phytochemical, however its main active metabolite after absorption is 11-hydroxy-Δ9-tetrahydroxannabinol (11-OH-THC), which has higher therapeutic potency (24, 29) and blood brain barrier (BBB) permeability. CBD is an antagonist of cannabinoid receptor agonists, and may offset the psychoactivity of THC when co-administered - reducing symptoms such as paranoia, dysphoria and anxiety (30–35) while also potentiating cannabis tolerability (36, 37). CBD is anti-inflammatory (38), but also exhibits other diverse effects including neuroprotective (39), anticonvulsive (40), antipsychotic (27, 41–43), anxiolytic (44), antidepressant (45), hypnotic, sedative, anticancer (46–51), analgesic and antiemetic activity (27). The anticancer properties of cannabinoids were first proposed in 1975 when Munson et al. (52) demonstrated THC and the related compound delta 8-THC as well as cannabinol (CBN) (but not CBD) reduced primary cancer growth through in vitro and in vivo lung adenocarcinoma growth murine models. Recent reviews have concluded that a large number of cannabinoid compounds have been discovered, developed, and used for cancer either alone or in combination with standard anti-cancer strategies. From these reviews, MC could become a therapy of choice in contemporary oncology (53) however, the tolerability of high dose THC has not been conducted in this brain cancer cohort.
Gliomas are the most common primary intracranial tumour of the central nervous system, accounting for one third of all brain tumours (54). Glioblastoma multiforme (GBM) is the most aggressive form of human brain tumour and are normally incurable due to the high recurrence rate, even after total resection (55, 56), with a median survival rate of approximately one year (57). Treatment of GBMs and high-grade gliomas such as anaplastic astrocytoma grade III (AA3)’s is challenged by their location, aggressive biological behaviour, diffuse infiltrative growth and low survival rate (58). Standard GBM and high-grade glioma (HGG) treatment involves surgical resection followed by adjuvant radiation therapy and chemotherapy (55). Non-clinical studies which have examined the effects of MC on high grade gliomas have been summarised in two systematic reviews conducted by Rocha et al. (59) and Rodriguez-Almaraz et al. (60). The 2014 review identified several key findings including details of the cellular and molecular mechanisms through which cannabinoids impact high grade gliomas. It reported evidence that CBD inhibits tumour angiogenesis, decreases the risk of metastasis and induces tumour cell apoptosis (59). The 2020 review found that there was limited moderate-quality evidence that supports the use of cannabinoids as an adjuvant to the standard care in the treatment of brain tumours and suggests that further prospective trials be conducted (60).
Clinical trials investigating the efficacy of MC for cancer have mainly examined cancer-related symptoms with limited trials focusing on cancer. One 2006 pilot trial involving patients with recurrent GBM (61) examined intra-tumour administration of THC on nine patients and reported a good safety profile (no significant changes in physical, neurological, biochemical or haematological parameters were noted) and possible anti-proliferative activity on the tumour cells (61).
The tolerability of high dose THC in different patient groups and the impact of MC on standard treatment efficacy is currently unknown. Given the preliminary research examining MC in patients with GBM (61), and the poor prognosis associated with this disease, we designed a trial to assess the tolerability of THC containing cannabis products.
Patients and Methods
Primary Objective
To investigate the tolerability of two different ratios of oral medicinal cannabis oil in patients who have been diagnosed with high grade gliomas (GBM or astrocytoma AA3r) as an adjunct to standard treatment.
Study Design
A single-centre Phase II double-blind randomised clinical trial assessing the tolerability of two different cannabinoid ratios of oral MC oil on patients with high-grade gliomas was conducted.
Participants
The study population for this trial were patients diagnosed with a recurrent or inoperable high-grade glioma (GBM or astrocytoma AA3r). They were aged 18 years or older, willing to participate in a clinical trial using MC and had no history of substance abuse. Each patient was assessed for European Cooperative Oncology Group score for functional performance status (ECOG) and screened for cognitive function via the general practice cognitive screening test (GPCOG) before being enrolled. Baseline blood tests were taken to ascertain cannabis metabolite levels prior to enrolment. They were excluded if they were pregnant or breast feeding, had severe cognitive impairment, severe mental illness, non-English speaking, had a past history of major substance abuse, had an adverse event from past cannabis use, severe liver or kidney disease or had a past history of myocardial infarctions. In addition, if participants were required to have another craniotomy while participating in the trial, they were excluded from the study. The study was conducted at the Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, NSW Australia from November 2018 to December 2019. The participants diagnosis was confirmed by one of the participants clinicians or if not possible, the doctors at the Centre for Minimally Invasive Neurosurgery. The confirmation of diagnosis was based on magnetic resonance imagining (MRI) discs and histopathology results from biopsy and/or surgery. No molecular markers were used so a range of genetic variations including 06-Methylguanine-DNA Methyltransferase (MGMT) promoter methylation, deletions of genes and mutations (IDH1, IDH 2) were included. As a pilot trial to ascertain any specific information, it was decided that a pragmatic approach be used for this trial to inform the next phase.
The advisory board and all investigators approved the study protocol. All patients provided written informed consent before study interventions were initiated. All researchers carried out the clinical trial in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and all possess a current Good Clinical Practice (GCP) certificate.
Investigation Products
The investigational products were sourced through Bioceuticals Pty Ltd from Whistler Medical Marijuana Corporation (WMMC) and consisted of two standardised and quality-assured specially formulated oil-based organic whole plant extracts of cannabis based on a 1:1 and 4:1 ratio of THC : CBD (1:1 THC 4.6mg/ml:CBD 4.8mg/ml and 4:1 THC 15mg/ml:CBD 3.8mg/ml) compliant with Therapeutic Goods Order # 93 (62). The investigational products were specifically made to conform with Good Manufacturing Practice (GMP) and were tested for quality to comply with Therapeutics Goods Administration (TGA), the Australian Federal regulator, requirements. Patients were given a single dose at night before bed that was titrated up to tolerance starting at 0.20ml (maximum 5ml in one dose).
Outcomes
The primary outcome of this trial was assessed by side effects and the Functional Assessment of Cancer Therapy- Brain (FACT-Br) (63) from baseline to 12 weeks in addition to patient tolerability of the oils via a participant diary. The FACT-Br is a commonly used quality of life (QoL)questionnaire for cancer related issues with questions specific to patients with brain cancer (63). Secondary outcomes included treatment-related toxicity, blood safety markers, dose response and tumour growth over 12 weeks. The blood cannabinoid metabolites and endocannabinoid level analysis will also be conducted and presented at a later date.
Randomisation
The randomisation sequence was conducted by the principal investigator and the allocation was only known by one Bioceuticals staff member responsible for coordinating product labelling. All researchers, pharmacists, doctors, patients, and staff were blinded to the allocation until after all analysis was completed. The reason for blinding the interventions, even though one was not a placebo, is because they both contained THC and the researchers wanted to minimize the possible effects of experimenter bias. A researcher being able to identify the intervention could potentially result in the researchers unknowingly influencing the results during the administration of the trial, data collection or the analysis.
The randomisation sequence was conducted using Research Randomizer: https://www.randomizer.org/. The randomisation was conducted for two sets using 46 participants. Allocation was via an on-line data management system, Clinical Studio, with the allocation not known until the person had been entered as enrolled into the system.
Drug Administration
Each intervention did not have a pharmacological name on the product. Both interventions were displayed in plain bottles with trial labels on them. They were labelled with either Arm A or B to distinguish between the groups. All investigator products were kept in a Schedule 8 facility in the private hospital pharmacy and were dispensed upon presentation of a trial script. The research nurse explained the administration of the drugs, titration information, dosing regimen, and potential side effects to each participant. Upon dispensing the drug, the trained pharmacist also explained the administration, dose, and potential side effects. The trial nurse contacted the participants every two days to ensure any adverse effects, dosage and increase or decrease of dosage until tolerated dose was reached.
Procedures
Treatment was conducted for 12 weeks in conjunction with standard treatment. Follow-up appointments occurred at 4, 8 and 12 weeks. A baseline and 12-week MRI scan were conducted and analysed using the RANO criteria (64) by a single centre radiologist at iMed radiology. Blood pathology was taken at each time point and toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse events (NCI-CTC: version 4.0) (65). The patients completed the FACT-Br (63) at each time point.
The MRIs were conducted with contrast in a 3T Phillips Dual Weighted-MRI scanner using single-shot spinecho EPI, axial and free breathing techniques at the IMed Prince of Wales Private Hospital. The blood pathology was collected and analysed by New South Wales (NSW) Health Pathology at baseline, 4 weeks, 8 weeks, and 12 weeks. Each blood test included electrolytes, liver function, kidney function, basal serology, C reactive protein, full blood count and if the participant was on phenytoin or carbamazepine, they were also tested each collection.
Retrospective Data Control Group
A retrospective control group acted as the historical control. The selection of the historical controls was matched for gender, age, disease state, MRI scans (12 weeks apart) and treatment. All historical controls were screened for no medicinal cannabis use during this time. This will be verified by what the doctor documented for other use and that it didn’t include medicinal cannabis.
A retrospective group was chosen to compliment the tolerability study of two different ratios of medicinal cannabis. Due to the absence of a prospective control group and comparing one ratio to another, the researchers decided it would be beneficial to have a comparison, hence the retrospective group was chosen.
The historical controls were patients of Dr Teo’s and his fellows. Medical records were accessed at the Centre of Minimally Invasive Neurosurgery, at the Prince of Wales Hospital, Randwick. The research nurse had access to the medical records at the Centre, and completed the Retrospective Data Collection Form (RDCF). No information on the form was identifiable and the medical records were not taken from the Centre.
Sample Size Calculation
No published study was found to be similar to this trial in terms of oral administration of the intervention, outcome measure, dosage of the intervention or time frame. Therefore, the sample size calculation was based on a trial conducted to examine high grade gliomas that used the standard deviation for the FACT-Br which is the primary outcome for this trial (66). To detect a difference between the intervention group and the control group of a change in FACT-Br (SD=8) with 80% power for 5% significance, we were required to recruit 82 participants (41 per arm). This allowed for a 20% attrition rate.
Statistical Analysis
Stata Version 14 was utilised to analyse all data using an intention-to-treat (ITT) basis. For the primary outcome measure, paired T tests and longitudinal mixed model analysis including mixed methods and generalized estimating equation [GEE] was used, with adjustments for potential confounders, including age and gender. Measures of safety and adverse events were analysed using the GEE to assess differences over time. Comparison to the retrospective historical data was conducted using unpaired t-tests. Evaluation of tumour growth was assessed using the RANO criteria from baseline to 12 weeks comparing the two arms. Statistical significance was set at p<0.05.
Ethics
The trial was approved by three human research ethics committees (HREC): South Eastern Sydney Local Health District HREC: 18/028, University of Technology Sydney HREC: ETH 18-2761 and Endeavour College of Natural Medicine HREC: 20180821.
Results
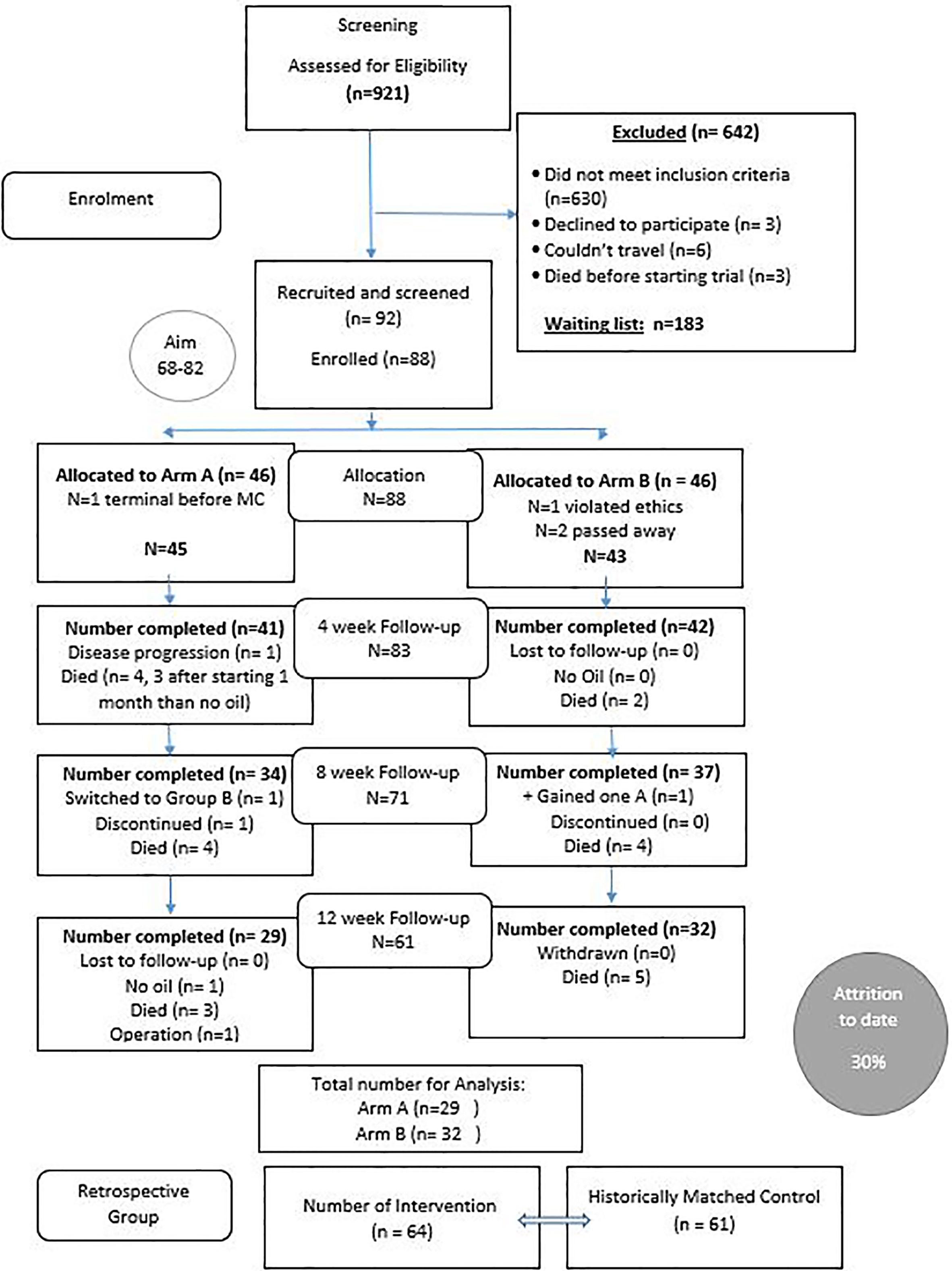
Nine hundred and twenty-one patients were initially screened for this trial due to a media release. Of those, 642 were excluded due to not meeting the inclusion criteria (many had brain metastasis or other brain cancers: see Figure 1) and 92 were recruited from around Australia and further screened by the study medical doctor (MS) for liver and kidney function and review of MRI results in addition to the research nurse screening for cognition. Eight-eight (88) participants were enrolled with 61 completing the 12-week intervention. As anticipated, an attrition rate of 30% occurred with 27 (29%) of the 92 dying before or during the trial. (see Figure 1).
Patient Characteristics
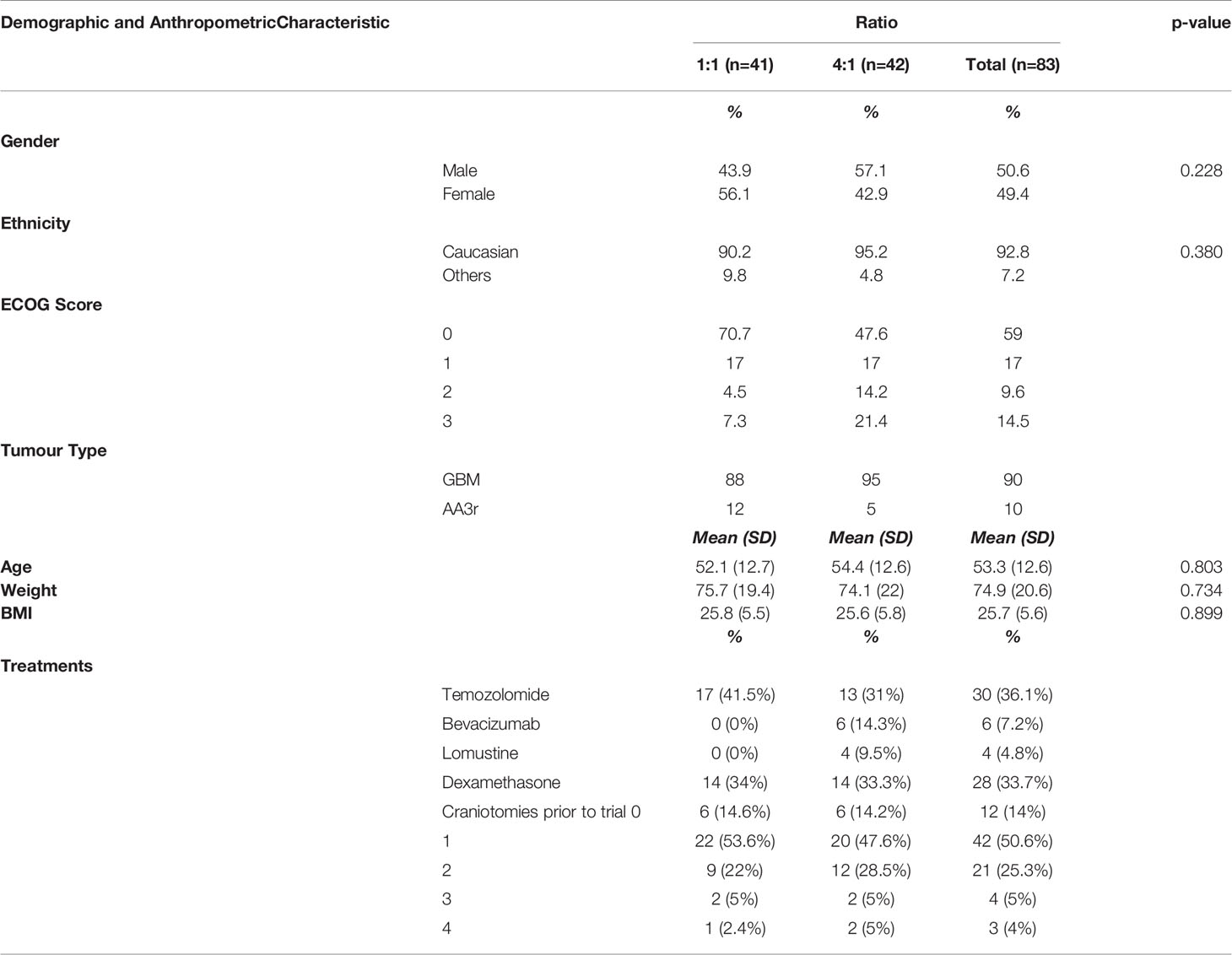
Ninety-two (92) patients were recruited and screened with 88 participants enrolled and 83 completing at least 4 weeks of the intervention (1:1 ratio n=41; 4:1 ratio n=42). The majority were Caucasian with a similar breakdown between females and males (1:1 female 56%, males 44%; 4.1 females 43%, males 57%). The average age of both groups was 53.3 years with an average BMI of 25.7. Thirty (n=30) participants were taking concurrent temozolomide (1:1, 41%; 4:1, 31%), four (4) participants were prescribed both Lomustine and Bevacizumab (1:1, 0%; 4:1, 9.5%) and two (2) prescribed Bevacizumab only (1;1, 0%; 4:1 14.3%). Of the participants, 28 had been administered dexamethasone (1:1, 34%; 4:1 33.3%), 14% had in-operatable GBM’s (1:1, 14.6%; 4:1, 14.2%), 50.6% had one craniotomy (1:1, 53.6%; 4:1, 47.6%) and 34.3% had 2 or more craniotomies (1:1, 29%; 4:1, 38.5%). (see Table 1).
Of the 92 patients screened, 91.3% (n=84) had a diagnosed recurrent or inoperable GBM brain tumour and 8.7% (n=8) had diagnosed AA3 recurrent. The ECOG scores from the 88 participants enrolled included 61.4% (n=54) having a score of 0, 17% (n=15) had a score of 1, 10.2% (n=9) had a score of 2 and 13.4% (n=12) had a score of 3 due to all subjects being in a wheelchair. No participants had a score of 4 on the ECOG scale.
Tolerability Results
The primary outcome of this trial was tolerability and both cannabis product ratios were found to be well tolerated. Three (3.4%) participants had their dose reduced due to side effects which included shaking and hallucinations at night with resolution of side effects following dose reduction [1:1 reduced from 3.8ml (THC 17.5mg:CBD 18.2mg) to 3ml (THC 13.8mg:CBD 14.4mg); 1:1 reduced from 2.1 (THC 9.7mg:CBD 10mg) to 1.5ml (THC 6.9mg:CBD 7.2mg); 4:1 reduced from 2.6ml (THC 39mg:CBD 9.9mg) to 1.5ml (THC 22.5mg:CBD 5.7mg) to 0.5ml (THC 7.5mg:CBD 1.9mg)].
The average dose tolerated in the 1:1 ratio was 2.25ml daily (THC 10.35mg:CBD 10.8mg), and for the 4:1 the average dose was 1.8ml daily (THC 27mg:CBD 6.8mg). Blood samples for each time point were collected and are currently being analysed for cannabinoids, cannabinoid metabolites and endocannabinoids to ensure compliance and evaluate contamination.
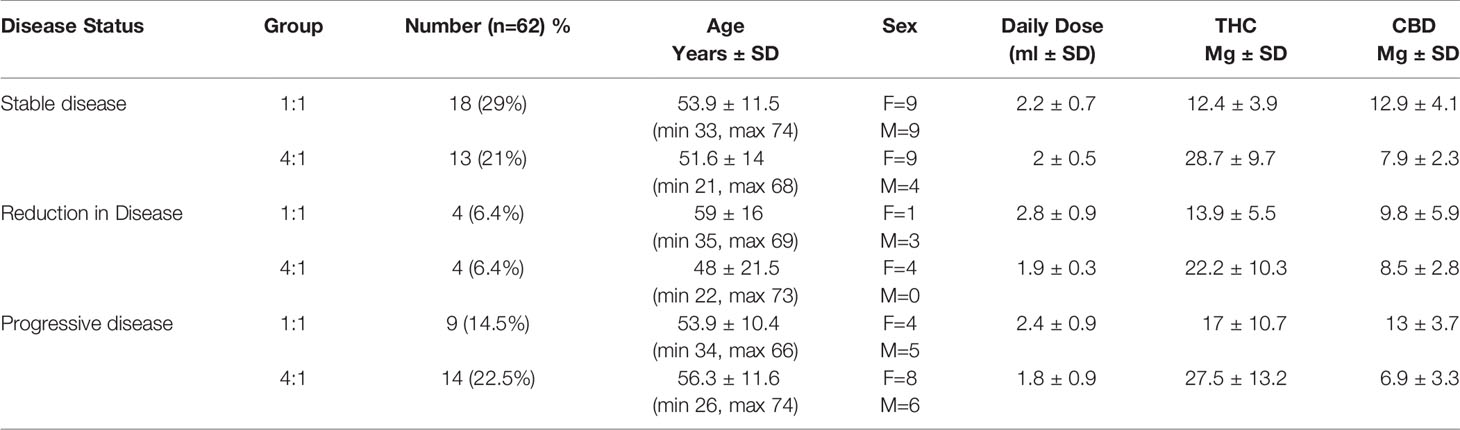
Expanding the analysis to the dose and amount of the tolerated cannabis compared to treatment outcome from the MRI results found that the participants with stable disease over the study period were equal numbers of females (n=9) and males (n=9) around 54 years old, and were primarily using the 1:1 ratio (n=18) with an average dose of 2.2ml daily (THC 12.4mg, CBD 12.9mg). For participants who had a reduction in disease, there were equal numbers for both the 1:1 (n=4) and 4:1 ratio (n=4). The 1:1 ratio had on average a dose of 2.8ml daily (THC 13.9, CBD 9.8) mostly male (n=3+ and around 59 years old. The 4:1 had on average a dose of 1.9ml daily (THC 22.2mg, CBD 8,.5mg), all female around 48 years of age. In the participant who had progressive disease, the majority were in the 4:1 ratio arm (n=14), had an average dose of 1.8ml daily (THC 27.5mg, CBD 6.9mg), and were mostly female (n=8) around 56 years old. (see Table 2)
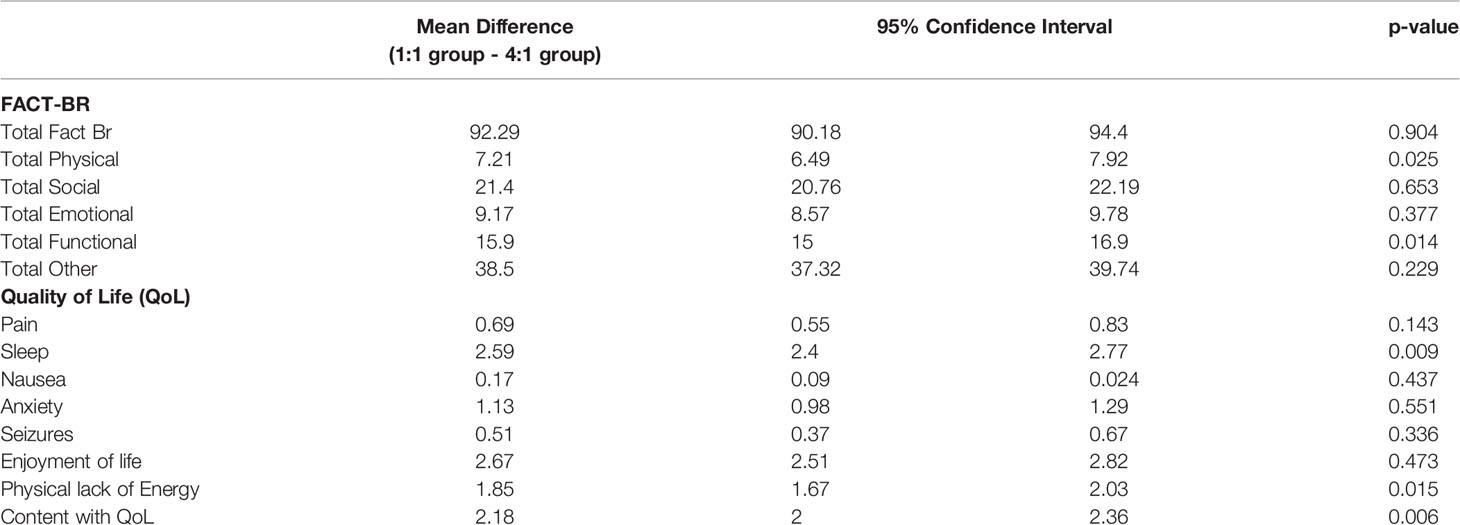
The results of the paired t-test assessing difference in the FACT-Br between groups from baseline to week 12 found that both physical (p=0.025) and functional (p=0.014) domains were statistically significant in favour of the 1:1 ratio over the 4:1 ratio. Sleep (p=0.009) improved in both groups. (see Table 3). A longitudinal model generalized estimating equation (GEE) was used to assess all patients over each time point and found both the physical wellbeing (p=0.045) and sleep (p=0.012) were statistically significant (see Table 4).
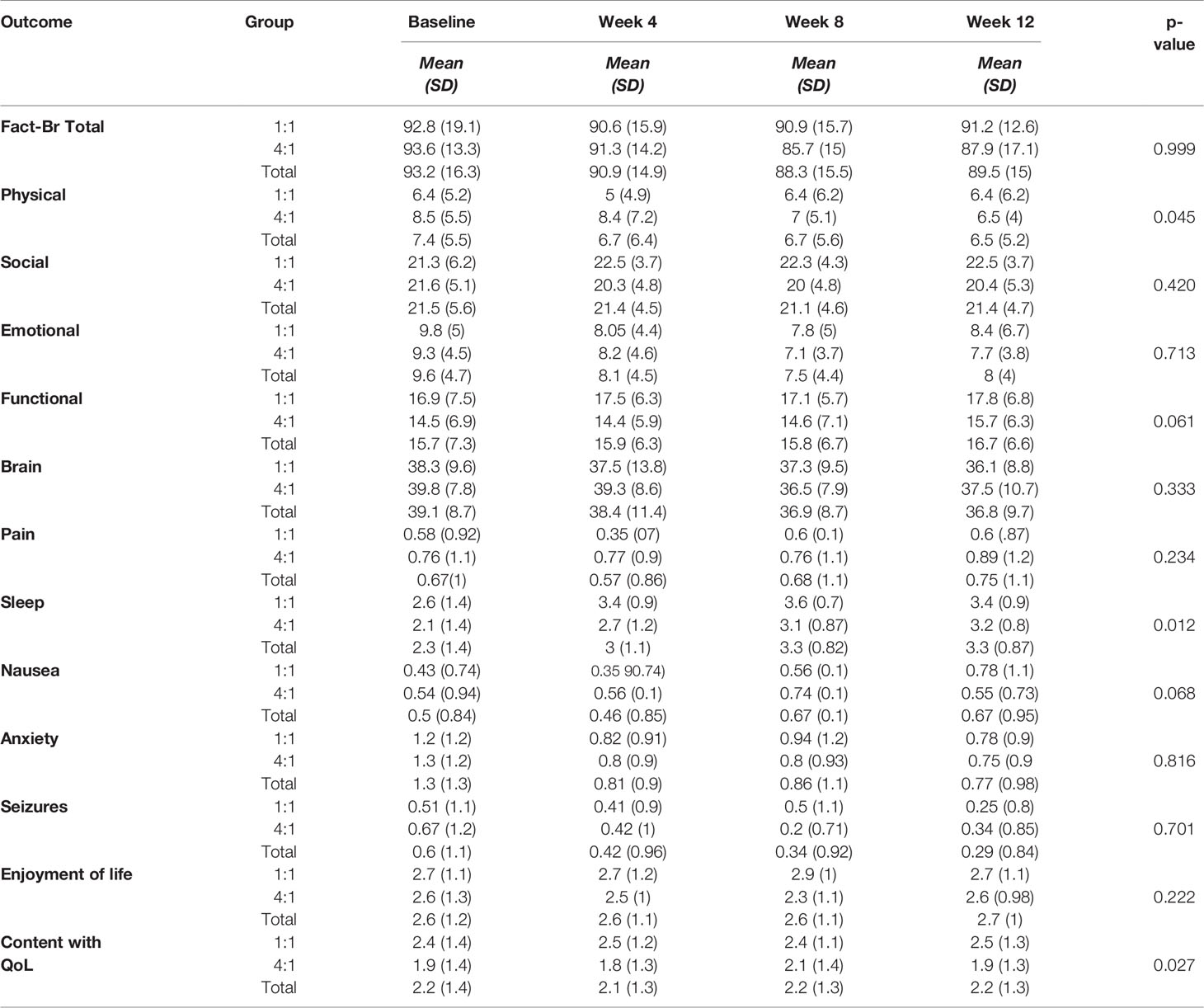
Table 4 Mean scores (based on GEE analyses) of the outcome measures for the study participants in the two treatment groups over time.
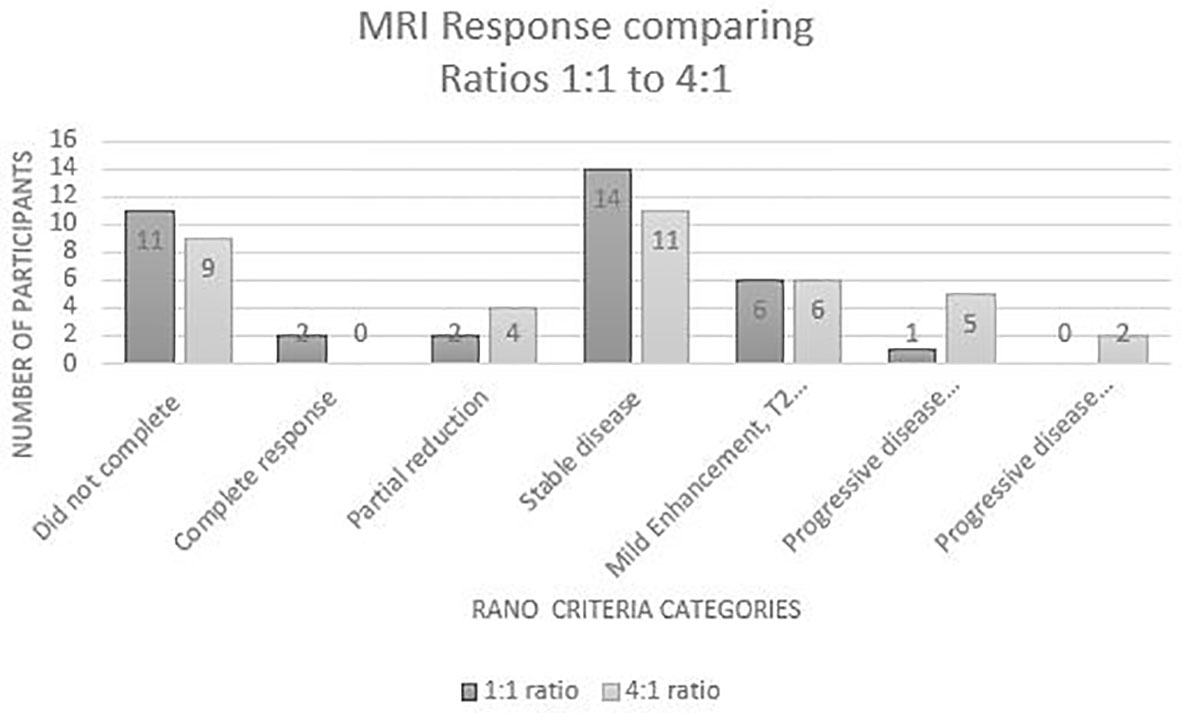
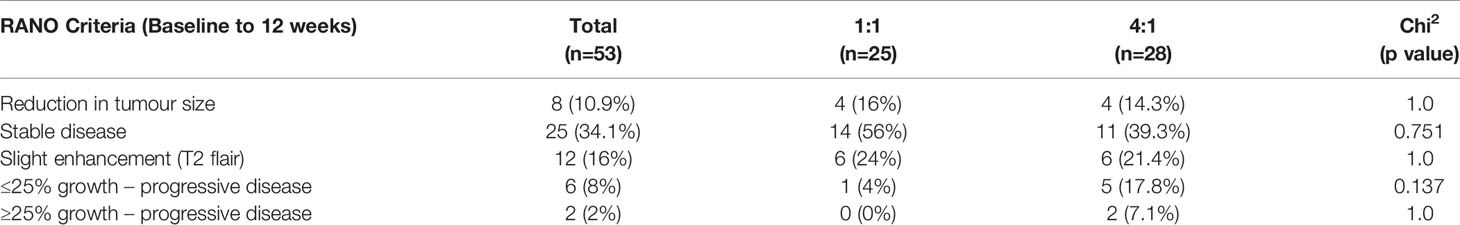
For the secondary outcome, disease status was assessed through comparison of MRI results. In accordance with the RANO criteria, the results of the participants who had an MRI at baseline and week 12 (n=53) identified that 11% had a reduction in disease, 34% had stable disease, 16% had a T2 flair and slight enhancement and 10% had progressive disease. (see Figure 2) A chi square analysis was conducted between groups for each disease status which found no statistical significance. Three of the disease states were the same value or too small to evaluate a difference. This indicates there is no difference between the 1:1 or the 4:1 for MRI tumour burden/control or reduction in relation cannabis ratios for disease status. (see Table 5).
Retrospective Control Group
The retrospective case data (n=61) included a greater proportion of dexamethasone administration (cannabis 33.7% vs 91% in retrospective data) and craniotomies in the control group compared with the study sample. The results of the cannabis group versus the retrospective data found 11% reduction in tumour size in cannabis arm versus 0% in retrospective data, 34% stable disease versus 45% stable disease, and 27.5% versus 53% for progressive disease.
Safety and Adverse Events
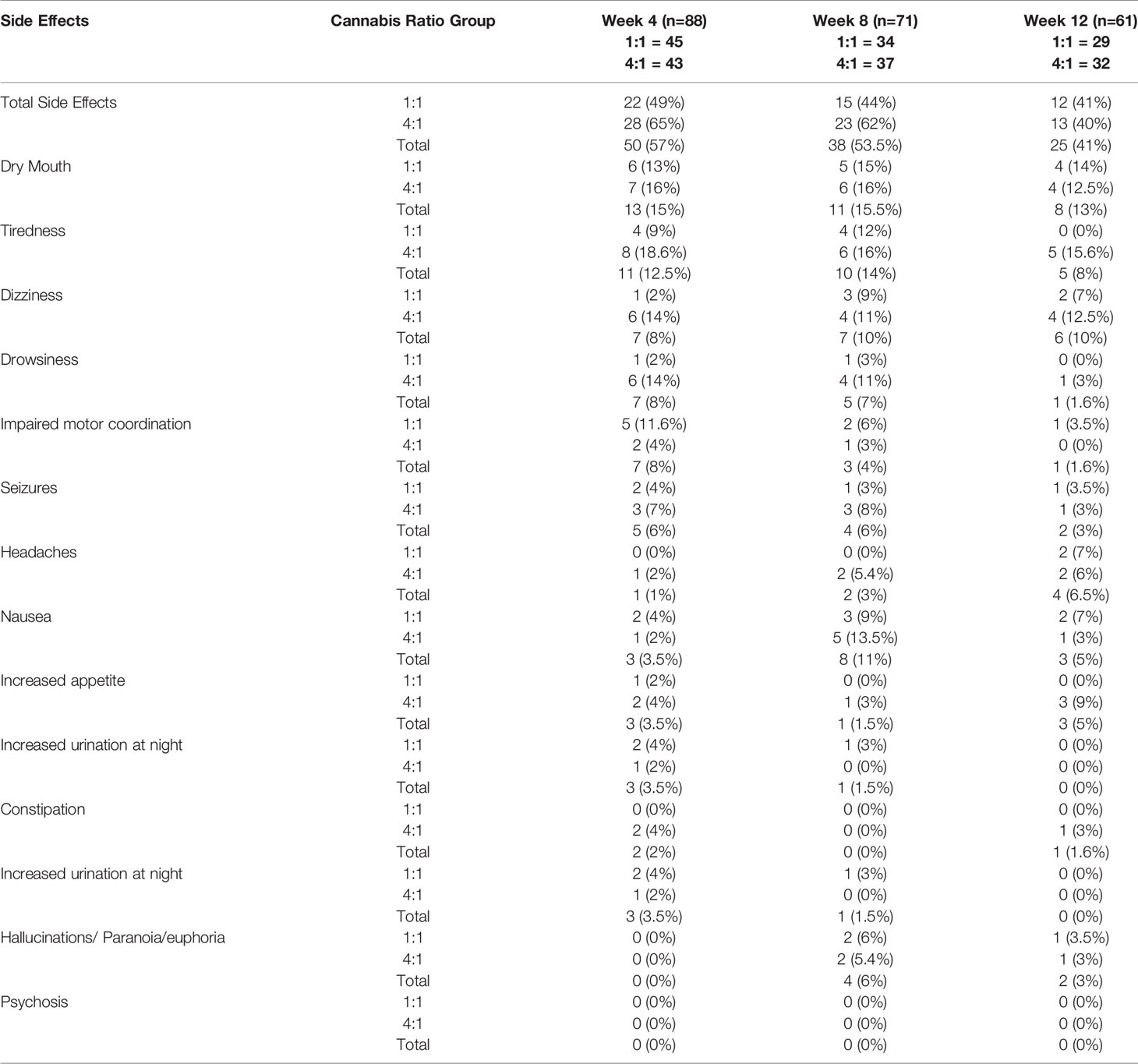
No serious adverse events were found to be attributable to the intervention during the trial. The main side effects noted were dry mouth (13%), tiredness at night (11%), dizziness mainly at night (10%), and drowsiness (7%). There were no reports of psychosis although at week 8, four participants (6%) reported mild hallucinations, some paranoia or euphoria at night. The number of total side effects identified decreased during the trial from 57% to 41% by week 12. (see Table 6).
The main findings from the NCI-CTC for all participants who completed the 12 weeks were bloating (p=0.034), shortness of breath (p=0.012), dry skin (p=0.034), visual floaters (p=0.037), concentration (p=0.033), muscle pain (p=0.019), and insomnia (p=0.011) (see Supplemental Information).
No statistically significant difference in blood pathology tests was identified between baseline to week 12 over time (see Supplemental Information). Serum liver enzymes were regularly checked via liver function tests (LFT) for all participants who were on specific treatments for high-grade gliomas. When associations between liver enzymes and the use of anticancer therapy were tested, a statistically significant association between raised GGT and temozolomide (n=30; p=0.028) was identified. Also, small number of participants (n=6) had been prescribed bevacizumab and a statistically significant association with elevated ALP and GGT was found in this group. Similarly, the analysis identified a statistically significant association between lomustine patients on Lomusine (n=4) and raised ALP (p=0.001).
Discussion
In this study, we performed a blinded, phase 2 trial administering MC to patients with high grade gliomas. Patients were randomized to one of two formulations with differing THC : CBD ratios. Currently, there are multiple studies explore the anti-inflammatory and anti-cancer potential of MC for patients with high grade gliomas. However, such research also relies on reliable information regarding tolerability and dosing before efficacy trials can be conducted. Currently this evidence is lacking in the literature.
The mechanism of action of CBD and THC is important for deciding which ratios are useful for particular diseases and symptoms (67). CBD is well known as the non-intoxicating cannabinoid compared to THC. However, it is the THC that binds to CB1 receptors which are mainly located in the central nervous system, predominately the brain, in addition to other organs and tissues (68). In vitro and animal studies have identified that cannabis has anti-tumoral activity and xenografts on rats and mice have shown that it can reduce high grade glioma growth (69, 70). Pellati et al. noted that both CBD and THC demonstrated anti-inflammatory activity by downregulation of the proto-oncogene c-fos and clycooxygenase-2 (COX-2) and potential anti-tumoral activity (71). Other in vitro models of neuroinflammation on rat microglia, found that CBD suppresses tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β and IL-6, reduces nuclear factor kappa beta (NF-κB) phosphorylation and activates COX and inducible nitric oxide synthase (iNOS) (72). CBD also causes a downregulation of Akt and ERK pathways in human glioma cells (73).
We propose this study provides robust evidence that medicinal cannabis administered to this patient population is safe, well tolerated, and can provide symptomatic relief to these patients, improving their QoL. While the overall blood brain barrier (BBB) penetration and general safety of cannabis has been demonstrated in healthy populations (74), this study is one of the first to evaluate the safety and tolerability of MC in this vulnerable cancer population, a patient group with BBB disruption, impaired brain function, and numerous medications including chemotherapy, corticosteroids, and antiepileptics.
Our study data suggests that cannabis, especially a 1:1 CBD/THC mixture can be helpful for many of the symptoms impacting QoL in this patient population, especially sleep disturbance. As such, MC may be a valuable potential therapy for maintaining the best QoL and daily function for this poor prognosis population, whist also assisting patients during anticancer and potential life extending therapies. High grade brain tumours, and their treatments, are well known to cause a constellation of symptoms, especially nausea from radiotherapy and chemotherapy, cerebral oedema or inflammation (75), increased intracranial pressure and sleep disturbance (or insomnia). This in part may be due to medication related side effects, notably corticosteroids in addition to radiation and chemotherapy (76). QoL concerns in patients with malignant high grade gliomas are relevant to more than just palliative care physicians, but have real oncologic and survival consequences (77).
Given the inevitability of tumour recurrence in these patients (78), patients with poorer QoL, higher symptom burdens and poorer functional status are less likely to elect for additional therapy, which often can extend their life (76). In this context, our study provides an important platform for further trials to explore the 1:1 mixture in larger cohorts.
Future Directions
This trial allowed a mixture of patients with different tumour grades, different disease stages (advanced, recurrent tumours and inoperable tumours), and did not sub-stratify patients by molecular markers, such as IDH-1 mutation. This style of recruitment was conducted to gather data for further trials to ascertain which cohort will be best to focus on for efficacy trials. The tolerability and improved symptomatic benefit for these patients encourages the incorporation of MC in future well designed, randomized-controlled trials that include cannabis-free controls, for the treatment of patients with high grade gliomas.
It is also important to note, that while generally we did not identify any adverse events related to drug interactions between MC and drugs commonly administered to high-grade glioma patients, notably chemotherapy and antiepileptics, we cannot exhaustively conclude that adding cannabis to the regimen of glioma patients will never lead to adverse drug interactions. Notably, the number of coadministration of MC with bevacizumab and lomustine was too low to meaningfully conclude this combination is safe in these patients. However, despite this limitation we feel this study supports the idea that it is reasonably safe to explore this further in this patient cohort.
Limitations
There were several limitations. Firstly, there was no placebo group which is considered gold standard in randomised-clinical trials and the historical retrospective data which was used as a comparison was compromised as the client population for the clinician providing the retrospective sample were commonly in advanced stages of cancer and hence many were excluded from the analysis. The difference in treatments used in the retrospective cases and the study population also limits the comparison between these groups. The length of time of the intervention (12 weeks) also limits the conclusions which can be drawn from this study and future research should consider longer time frames. However, longitudinal studies are continuing for this trial with up to two years follow up of the cohort. This trial was also very pragmatic, rather than controlled, to give as broad a clinically useful picture as possible. As such, this limits the strength of the data due the heterogeneity of the cohort and the variations in concurrent treatments. In saying that, real life pragmatic trials are necessary for translation and implementation and can give clinicians a much clearer patient picture for everyday care. In addition, a strong intervention affect can be expected for the MC group as there was no placebo. It would be advised that a placebo-controlled trial be conducted in the future to ascertain accurate data.
Conclusion
Addressing the symptom clusters, improving symptoms and in turn QoL is the first and crucial step in improving health outcomes for this population of cancer patients. This remains a vulnerable patient group with rapid deterioration, poor QoL and poor prognosis. Despite increasing interest in the efficacy in disease control of MC in this population, associated research examining the tolerability and safety of MC products is scarce. From this study we have shown that a single nightly dose of THC-containing cannabis was well tolerated in patients in both groups with high-grade gliomas and significantly improved sleep, functional wellbeing and contentment with QoL in a sample of patients compared to baseline. From this trial, the 1:1 ratio has been identified as the preferred combination the moving forward to further trials. This study significantly informs MC product choice for ongoing studies into cannabis being a potential adjunct treatment option for this patient population.
Study Sponsors
Endeavour College of Natural Health, 269 Wickham Street, Fortitude Valley, Qld 4006 NCNM, Southern Cross University, Military Road, Lismore NSW. NB: The funding body was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Data Availability Statement
The raw data supporting the conclusions of this article can be made available upon request by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by South Eastern Sydney Local Health District HREC. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JaS was the lead for the manuscript with all authors contributed to the paper in addition to editing final versions. All authors contributed to the article and approved the submitted version.
Funding
This study was supported financially by FIT-Bioceuticals Pty Ltd, Sydney, Australia. The funder bodies were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All participants and their families for being part of the trial. Bioceuticals for funding and supplying the investigational product for the trial. The investigational product was manufactured by Whistler Medical Marijuana Company, Canada. The Centre for Minimally Invasive Neurosurgery staff for conducting the trial in their offices.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.649555/full#supplementary-material
References
1. Zogopoulos P KP, Patsouris E, Theocharis S. The Antitumour Action of Cannabinoids on Glioma Tumorigenesis. Histol Histopathol (2015) 30:629–45. doi: 10.14670/HH-30.629
2. National Academies of Sciences, Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids. In: The Current State of Evidence and Recommendations for Research. Washington DC: The National Academies Press (2017).
3. Pinkas J JP, Kidawa M, Wierzba W. Use of Marijuana for Medical Purposes. Ann Agric Environ Med (2016) 23(3):525–8. doi: 10.5604/12321966.1219200
4. Wall MM, Liu J, Hasin DS, Blanco C, Olfson M. Use of Marijuana Exclusively for Medical Purposes. Drug Alcohol Depend (2019) 195:13–5. doi: 10.1016/j.drugalcdep.2018.11.009
5. Giacoppo S, Mandolino G, Galuppo M, Bramanti P, Mazzon E. Cannabinoids: New Promising Agents in the Treatment of Neurological Diseases. Mol (Basel Switzerland) (2014) 19(11):18781–816. doi: 10.3390/molecules191118781
6. Hanus LO, Meyer SM, Munoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: A Unified Critical Inventory. Nat Prod Rep (2016) 33(12):1357–92. doi: 10.1039/C6NP00074F
7. Berman P, Futoran K, Lewitus GM, Mukha D, Benami M, Shlomi T, et al. A New ESI-LC/MS Approach for Comprehensive Metabolic Profiling of Phytocannabinoids in Cannabis. Sci Rep (2018) 8(1):14280. doi: 10.1038/s41598-018-32651-4
8. Shelef A BY, Berger U, Paleacu D, Tadger S, Plopsky I, Baruch Y. Safety and Efficacy of Medical Cannabis Oil for Behavoural and Psychological Symptoms of Dementia: An-Open Label, Add-On, Pilot Study. J Alzheimer’s Dis (2016) 51:15–9. doi: 10.3233/JAD-150915
9. Rahn EJ, Hohmann AG. Cannabinoids as Pharmacotherapies for Neuropathic Pain: From the Bench to the Bedside. Neurother J Am Soc Exp NeuroTher (2009) 6(4):713–37. doi: 10.1016/j.nurt.2009.08.002
10. Aggarwal SK. Cannabinergic Pain Medicine: A Concise Clinical Primer and Survey of Randomized-Controlled Trial Results. Clin J Pain (2013) 29(2):162–71. doi: 10.1097/AJP.0b013e31824c5e4c
11. Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc Natl Acad Sci USA (1998) 95(14):8268–73. doi: 10.1073/pnas.95.14.8268
12. Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized Controlled Trial of Sativex to Treat Detrusor Overactivity in Multiple Sclerosis. Mult Scler (2010) 16(11):1349–59. doi: 10.1177/1352458510378020
13. Williams SJ, Hartley JP, Graham JD. Bronchodilator Effect of delta1-tetrahydrocannabinol Administered by Aerosol of Asthmatic Patients. Thorax (1976) 31(6):720–3. doi: 10.1136/thx.31.6.720
14. Neff GW, O’Brien CB, Reddy KR, Bergasa NV, Regev A, Molina E, et al. Preliminary Observation With Dronabinol in Patients With Intractable Pruritus Secondary to Cholestatic Liver Disease. Am J Gastroenterol (2002) 97(8):2117–9. doi: 10.1111/j.1572-0241.2002.05852.x
15. Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic Activity of Cannabinoids. J Natl Cancer Institute (1975) 55(3):597–602. doi: 10.1093/jnci/55.3.597
16. Sanchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Delta9-tetrahydrocannabinol Induces Apoptosis in C6 Glioma Cells. FEBS Lett (1998) 436(1):6–10. doi: 10.1016/S0014-5793(98)01085-0
17. Blazquez C, Salazar M, Carracedo A, Lorente M, Egia A, Gonzalez-Feria L, et al. Cannabinoids Inhibit Glioma Cell Invasion by Down-Regulating Matrix Metalloproteinase-2 Expression. Cancer Res (2008) 68(6):1945–52. doi: 10.1158/0008-5472.CAN-07-5176
18. Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, et al. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells Via Endoplasmic Reticulum Stress-Related Genes. Cancer Res (2006) 66(13):6748–55. doi: 10.1158/0008-5472.CAN-06-0169
19. Ruiz L, Miguel A, Diaz-Laviada I. Delta9-tetrahydrocannabinol Induces Apoptosis in Human Prostate PC-3 Cells Via a Receptor-Independent Mechanism. FEBS Lett (1999) 458(3):400–4. doi: 10.1016/S0014-5793(99)01073-X
20. Leelawat S, Leelawat K, Narong S, Matangkasombut O. The Dual Effects of Delta(9)-Tetrahydrocannabinol on Cholangiocarcinoma Cells: Anti-Invasion Activity At Low Concentration and Apoptosis Induction At High Concentration. Cancer Invest (2010) 28(4):357–63. doi: 10.3109/07357900903405934
21. Whyte DA, Al-Hammadi S, Balhaj G, Brown OM, Penefsky HS, Souid AK. Cannabinoids Inhibit Cellular Respiration of Human Oral Cancer Cells. Pharmacology (2010) 85(6):328–35. doi: 10.1159/000312686
22. Machado Rocha FC, Stefano SC, De Cassia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic Use of Cannabis Sativa on Chemotherapy-Induced Nausea and Vomiting Among Cancer Patients: Systematic Review and Meta-Analysis. Eur J Cancer Care (Engl) (2008) 17(5):431–43. doi: 10.1111/j.1365-2354.2008.00917.x
23. Russo EB. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br J Pharmacol (2011) 163(7):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x
24. Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A. Cannabinoids. J Pain Symptom Manage (2013) 46(1):142–9. doi: 10.1016/j.jpainsymman.2013.05.002
25. Gaoni Y, Mechoulam R. The Isolation and Structure of delta-1-tetrahydrocannabinol and Other Neutral Cannabinoids From Hashish. J Am Chem Soc (1971) 93(1):217–24. doi: 10.1021/ja00730a036
26. Pertwee RG. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: delta9-tetrahydrocannabinol, Cannabidiol and Delta9-Tetrahydrocannabivarin. Br J Pharmacol (2008) 153(2):199–215. doi: 10.1038/sj.bjp.0707442
27. Upton R, ElSohly M, Romm A, Russo E, Sexton M, eds. Cannabis Inflorescence. Scotts Valley, California: American Herbal Pharmacopoeia (2013).
28. Howlett AC, Blume LC, Dalton GD. CB(1) Cannabinoid Receptors and Their Associated Proteins. Curr Med Chem (2010) 17(14):1382–93. doi: 10.2174/092986710790980023
29. Grotenhermen F. Cannabinoids and the Endocannabinoid System. International Association for Cannabis as Medicine. Cannabinoids (2006) 1(1):10–4.
30. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol Interferes With the Effects of Delta 9 - Tetrahydrocannabinol in Man. Eur J Pharmacol (1974) 28(1):172–7. doi: 10.1016/0014-2999(74)90129-0
31. Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of Cannabidiol on delta-9-tetrahydrocannabinol Effects. Clin Pharmacol Ther (1976) 19(3):300–9. doi: 10.1002/cpt1976193300
32. Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite Effects of delta-9-tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology (2010) 35(3):764–74. doi: 10.1038/npp.2009.184
33. Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol Inhibits THC-elicited Paranoid Symptoms and Hippocampal-Dependent Memory Impairment. J Psychopharmacol (Oxford England) (2013) 27(1):19–27. doi: 10.1177/0269881112460109
34. Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, et al. Diminished Gray Matter in the Hippocampus of Cannabis Users: Possible Protective Effects of Cannabidiol. Drug Alcohol Dependence (2011) 114(2-3):242–5. doi: 10.1016/j.drugalcdep.2010.09.020
35. Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of Cannabidiol on the Anxiety and Other Effects Produced by Delta 9-THC in Normal Subjects. Psychopharmacology (1982) 76(3):245–50. doi: 10.1007/BF00432554
36. Karniol IG, Carlini EA. Pharmacological Interaction Between Cannabidiol and Delta 9-Tetrahydrocannabinol. Psychopharmacologia (1973) 33(1):53–70. doi: 10.1007/BF00428793
37. Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: Pharmacology and Potential Therapeutic Role in Epilepsy and Other Neuropsychiatric Disorders. Epilepsia (2014) 55(6):791–802. doi: 10.1111/epi.12631
38. Mukhopadhyay P, Rajesh M, Horvath B, Batkai S, Park O, Tanchian G, et al. Cannabidiol Protects Against Hepatic Ischemia/Reperfusion Injury by Attenuating Inflammatory Signaling and Response, Oxidative/Nitrative Stress, and Cell Death. Free Radic Biol Med (2011) 50(10):1368–81. doi: 10.1016/j.freeradbiomed.2011.02.021
39. Sanchez AJ, Garcia-Merino A. Neuroprotective Agents: Cannabinoids. Clin Immunol (2012) 142(1):57–67. doi: 10.1016/j.clim.2011.02.010
40. Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol Displays Antiepileptiform and Antiseizure Properties In Vitro and In Vivo. J Pharmacol Exp Ther (2010) 332(2):569–77. doi: 10.1124/jpet.109.159145
41. Zuardi AW, Hallak JE, Dursun SM, Morais SL, Sanches RF, Musty RE, et al. Cannabidiol Monotherapy for Treatment-Resistant Schizophrenia. J Psychopharmacol (Oxford England) (2006) 20(5):683–6. doi: 10.1177/0269881106060967
42. Morgan CJ, Curran HV. Effects of Cannabidiol on Schizophrenia-Like Symptoms in People Who Use Cannabis. Br J Psychiatry (2008) 192(4):306–7. doi: 10.1192/bjp.bp.107.046649
43. Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Trans Psychiatry (2012) 2:e94. doi: 10.1038/tp.2012.15
44. Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naive Social Phobia Patients. NeuropsychoPharmacology (2011) 36(6):1219–26. doi: 10.1038/npp.2011.6
45. Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR. Antidepressant-Like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT1A Receptors. Br J Pharmacol (2010) 159(1):122–8. doi: 10.1111/j.1476-5381.2009.00521.x
46. Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor Activity of Plant Cannabinoids With Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma. J Pharmacol Exp Ther (2006) 318(3):1375–87. doi: 10.1124/jpet.106.105247
47. Solinas M, Massi P, Cantelmo AR, Cattaneo MG, Cammarota R, Bartolini D, et al. Cannabidiol Inhibits Angiogenesis by Multiple Mechanisms. Br J Pharmacol (2012) 167(6):1218–31. doi: 10.1111/j.1476-5381.2012.02050.x
48. Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol Inhibits Human Glioma Cell Migration Through a Cannabinoid Receptor-Independent Mechanism. Br J Pharmacol (2005) 144(8):1032–6. doi: 10.1038/sj.bjp.0706134
49. Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk Between Apoptosis and Autophagy. Mol Cancer Ther (2011) 10(7):1161–72. doi: 10.1158/1535-7163.MCT-10-1100
50. McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Mol Pharmacol (2006) 70(3):897–908. doi: 10.1124/mol.106.023937
51. Brown I, Cascio MG, Rotondo D, Pertwee RG, Heys SD, Wahle KW. Cannabinoids and omega-3/6 Endocannabinoids as Cell Death and Anticancer Modulators. Prog Lipid Res (2013) 52(1):80–109. doi: 10.1016/j.plipres.2012.10.001
52. Munson AE HL, Friedman MA, Dewey WL, Carchman RA. Antineoplastic Activity of Cannabinoids. J Natl Cancer Inst (1975) 55(3):597–602. doi: 10.1093/jnci/55.3.597
53. Bogdanović V, Mrdjanović J, Borišev I. A Review of the Therapeutic Antitumor Potential of Cannabinoids. J Altern Complement Med (2017) 23(11):831–6. doi: 10.1089/acm.2017.0016
54. Jin Z, Jin RH, Ma C, Li HS, Xu HY. Serum Expression Level of miR-504 can Differentiate Between Glioblastoma Multiforme and Solitary Brain Metastasis of non-Small Cell Lung Carcinoma. J BUON Off J Balkan Union Oncol (2017) 22(2):474–80.
55. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
56. Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. David: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol (2003) 4(5):P3. doi: 10.1186/gb-2003-4-5-p3
57. Wilson TA KM, Harter DH. Glioblastoma Multiforme: State of the Art and Future Therapeutics. Surg Neurol Int (2014) 5:64. doi: 10.4103/2152-7806.132138
58. Kesari S. Understanding Glioblastoma Tumor Biology: The Potential to Improve Current Diagnosis and Treatments. Semin Oncol (2011) 38(Suppl 4):S2–10. doi: 10.1053/j.seminoncol.2011.09.005
59. Rocha FCMd, Stefano SC, da Silveira DX. Systematic Review of the Literature on Clinical and Experimental Trials on the Antitumor Effects of Cannabinoids in Gliomas. J Neurooncol (2014) 116:11–24. doi: 10.1007/s11060-013-1277-1
60. Rodriguez-Almaraz JE, Chang S, Clarke J, Oberheim-Bush NA, Taylor J, Buerki R, et al. A Systematic Review and Meta-Analysis Examining the Effects of Cannabis and its Derivatives in Adults With Malignant CNS Tumors. Neurooncol Pract (2020) 7(4):376–83. doi: 10.1093/nop/npaa013
61. Guzmán M DM, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sánchez C, et al. A Pilot Clinical Study of Delta9-tetrahydrocannabinol in Patients With Recurrent Glioblastoma Multiforme. Br J Cancer (2006) 95(2):197–203. doi: 10.1038/sj.bjc.6603236
62. Administration TG. Conforming With Therapeutic Goods (Standard for Medicinal Cannabis) TOG 93 Order 2017. Australia: Australian Government (2020). Available at: https://www.tga.gov.au/sites/default/files/conforming-tgo-93-standard-medicinal-cannabis.pdf.
63. Lien K, Zeng L, Nguyen J, Cramarossa G, Cella D, Chang E, et al. Fact-Br for Assessment of Quality of Life in Patients Receiving Treatment for Brain Metastases: A Literature Review. Expert Rev Pharmacoecon Outcomes Res (2011) 11(6):701–8. doi: 10.1586/erp.11.67
64. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response Assessment Criteria for Brain Metastases: Proposal From the RANO Group. Lancet Oncol (2015) 16(6):e270–8. doi: 10.1016/S1470-2045(15)70057-4
65. Health NIo. Common Terminology Criteria for Adverse Events v4.0 (Ctcae). In: Insitute NC, editor. United States of America: U.S. Department of Health and Human Services (2009). p. 1–79.
66. Piil K, Jakobsen J, Christensen KB, Juhler M, Jarden M. Health-Related Quality of Life in Patients With High-Grade Gliomas: A Quantitative Longitudinal Study. J Neurooncol (2015) 124(2):185–95. doi: 10.1007/s11060-015-1821-2
67. MacCallum CA, Russo EB. Practical Considerations in Medical Cannabis Administration and Dosing. Eur J Internal Med (2018) 49:12–9. doi: 10.1016/j.ejim.2018.01.004
68. Kis B, Ifrim FC, Buda V, Avram S, Pavel IZ, Antal D, et al. Cannabidiol-From Plant to Human Body: A Promising Bioactive Molecule With Multi-Target Effects in Cancer. Int J Mol Sci (2019) 20(23):5905. doi: 10.3390/ijms20235905
69. Velasco G, Sánchez C, Guzmán M. Towards the Use of Cannabinoids as Antitumour Agents. Nat Rev Cancer (2012) 12(6):436–44. doi: 10.1038/nrc3247
70. Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for Cancer Treatment: Progress and Promise. Cancer Res (2008) 68(2):339–42. doi: 10.1158/0008-5472.CAN-07-2785
71. Pellati F, Borgonetti V, Brighenti V, Biagi M, Benvenuti S, Corsi L, et al. And Nonpsychoactive Cannabinoids: Their Chemistry and Role Against Oxidative Stress, Inflammation, and Cancer. BioMed Res Int (2018) 2018:1691428. doi: 10.1155/2018/1691428
72. Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids Delta(9)-tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-Activated NF-kappaB and Interferon-Beta/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J Biol Chem (2010) 285(3):1616–26. doi: 10.1074/jbc.M109.069294
73. Solinas M, Massi P, Cinquina V, Valenti M, Bolognini D, Gariboldi M, et al. Cannabidiol, a non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells Through a Multitarget Effect. PloS One (2013) 8(10):e76918. doi: 10.1371/journal.pone.0076918
74. Wang T, Collet JP, Shapiro S, Ware MA. Adverse Effects of Medical Cannabinoids: A Systematic Review. CMAJ Can Med Assoc J J l’Assoc Med Canadienne (2008) 178(13):1669–78. doi: 10.1503/cmaj.071178
75. Jackson C, Westphal M, Quiñones-Hinojosa A. Complications of Glioma Surgery. Handb Clin Neurol (2016) 134:201–18. doi: 10.1016/B978-0-12-802997-8.00012-8
76. Pellerino A, Franchino F, Soffietti R, Ruda R. Overview on Current Treatment Standards in High-Grade Gliomas. Q J Nucl Med Mol Imaging (2018) 62(3):225–38. doi: 10.23736/S1824-4785.18.03096-0
77. Omuro A, DeAngelis LM. Glioblastoma and Other Malignant Gliomas: A Clinical Review. Jama (2013) 310(17):1842–50. doi: 10.1001/jama.2013.280319
Keywords: glioblastoma multiforme (GBM), cannabis (marijuana), medicinal marijuana, high-grade glioma (HGG), tolerability, quality of life
Citation: Schloss J, Lacey J, Sinclair J, Steel A, Sughrue M, Sibbritt D and Teo C (2021) A Phase 2 Randomised Clinical Trial Assessing the Tolerability of Two Different Ratios of Medicinal Cannabis in Patients With High Grade Gliomas. Front. Oncol. 11:649555. doi: 10.3389/fonc.2021.649555
Received: 05 January 2021; Accepted: 22 April 2021;
Published: 21 May 2021.
Edited by:
Emeline Tabouret, Aix Marseille Université, FranceReviewed by:
Quan Cheng, Central South University, ChinaVadim Kumeiko, Far Eastern Federal University, Russia
Copyright © 2021 Schloss, Lacey, Sinclair, Steel, Sughrue, Sibbritt and Teo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janet Schloss, janet.schloss@scu.edu.au; orcid.org/0000-0003-1643-6215
 Janet Schloss
Janet Schloss Judith Lacey4,5,6
Judith Lacey4,5,6 Michael Sughrue
Michael Sughrue Charles Teo
Charles Teo