
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 29 January 2018
Sec. Mucosal Immunity
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00055
Short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, modulate immune responses in the gut. However, the effect of SCFAs on mucosal vaccine-induced immune cell migration is poorly understood. Here, we investigated whether SCFAs modulate chemokine expression induced by the killed whole-cell oral cholera vaccine, Shanchol™, in human intestinal epithelial cells. Shanchol™ induced expression of CCL2, CCL5, CCL20, and CXCL10 at the mRNA level, but not at the protein level. Interestingly, CCL20 secretion was substantially increased by co-stimulation with Shanchol™ and butyrate, while neither acetate nor propionate showed such effect. Enhanced CCL20 secretion was associated with GPR109A activation, and histone deacetylase (HDAC) inhibition. In addition, co-treatment with Shanchol™ and butyrate synergistically increased the secretion of adenosine triphosphate (ATP). Moreover, CCL20 secretion was decreased by inhibiting the extracellular ATP receptor P2X7. However, neither inflammasomes nor caspases were involved in CCL20 production. The culture supernatant of cells treated with Shanchol™ and butyrate augmented human immature dendritic cell migration. Collectively, these results suggest that butyrate enhances Shanchol™-induced CCL20 production in human intestinal epithelial cells via HDAC inhibition and ATP-P2X7 signaling by activating GPR109A. These effects potentially enhance the mucosal immune responses in the gut induced by this oral cholera vaccine.
Cholera is an acute diarrheal disease that can cause life-threatening dehydration and shock if not treated appropriately. It is caused by ingestion of water or food contaminated with Vibrio cholerae (1). Currently, only killed whole-cell-based oral cholera vaccines are commercially available. Dukoral™ is formulated with heat-killed or formalin-killed V. cholerae O1 together with the recombinant cholera toxin B subunit (CTB) (2). Shanchol™ includes formalin-killed V. cholerae O139 and heat-killed or formalin-killed V. cholerae O1, but does not include the recombinant CTB subunit (3). Although both cholera vaccines have been successfully licensed, their low immunogenicity, short-term protection, and high dose requirement leave a room for further improvement (4, 5). Enhancement of intestinal mucosal immunity has been suggested to be one of the most efficient approaches by which the development of modern oral vaccines against cholera can be improved (6).
Mucosal immune responses induced by oral cholera vaccines are mainly mediated by anti-bacterial and anti-cholera toxin antibodies in the mucosal compartments of gastrointestinal tract (7). The gastrointestinal tract has gut-associated lymphoid tissues (GALTs), such as Peyer’s patches, which consist of antigen-presenting cells (APCs) and lymphocytes, both of which play a crucial role in the mucosal immune system (8). Following oral vaccination, antigens in the mucosal inductive site can be sampled by M cells and transported to APCs or directly captured by APCs such as dendritic cells (DCs). In Peyer’s patches, antigen-loaded DCs migrate to T-cell areas and subsequently present the antigen to T cells in GALTs and the mesenteric lymph nodes. Finally, IgA-producing plasmablasts home to the effector site, after which antigen-specific dimeric IgA antibodies are produced and transported to the lumen.
Epithelial cells in the gastrointestinal tract induce mucosal immune responses by producing immune mediators such as chemokines (9). Chemokines play a central role in the mucosal immunity by regulating the patterns of leukocyte chemotactic migration. For example, CCL25 is highly expressed in the small intestine where it supports lymphocyte homing (10). Besides, in the large intestine, CCL28, also called mucosa-associated epithelial chemokine, is expressed and plays a key role in the recruitment of IgA antibody-secreting cells (11). CCL20, also known as macrophage inflammatory protein-3α, binds to CC chemokine receptor-6 (CCR6) (12) and attracts immature DCs (13), memory T cells (14), and B cells (15). In addition, increased number of DCs induced by CCL20 upon mucosal vaccination is associated with the extent to which IgA and IgG levels in the nasal mucosa are elevated (16), indicating that chemokine induction is important for efforts to improve vaccine efficacy.
Short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, are the major metabolites of dietary fibers derived from the intestinal microbiota. These metabolites can regulate intestinal immune responses by enteric pathogens (17). SCFAs modulate immune cell function by inhibiting the activity of histone deacetylase (HDAC), which regulates epigenetic modification, or by activating G-protein-coupled receptors (GPCRs) (18–20), thus modulating chemokine production and release (21). Although SCFAs are the major end products of gut microbiota in the large intestine, they are also found in the small intestine (22). In addition, SCFAs increase the number of IgA+ plasma cells in the small intestine (23). Since microbial metabolites, including SCFAs in the gut, can affect mucosal vaccine efficacy (24), they would be employed as vaccine adjuvants possibly by being delivered together with vaccines, or by being elevated in the gut with high fiber diet prior to vaccination. However, the effects of SCFAs on mucosal vaccine-induced immune responses, particularly chemokine expression, are not clearly understood. In this study, we investigated whether SCFAs modulate Shanchol™-induced chemokine expression in human intestinal epithelial cells, with the aim of further improving vaccine efficacy.
The killed whole-cell oral cholera vaccine, Shanchol™, was purchased from Shantha Biotechnics (Hyderabad, India). Sodium acetate, sodium butyrate, sodium propionate, adenosine triphosphate (ATP), trichostatin A (TSA), mepenzolate bromide (MPN), and oxATP were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). SB203580, PD98059, and SP600125 were purchased from Calbiochem (La Jolla, CA, USA). For Western blot analysis, a monoclonal antibody specific to acetyl-histone H3 (Lys9) was obtained from Cell Signaling Technology (Beverly, MA, USA). For chromatin immunoprecipitation (ChIP) assay, a polyclonal antibody against acetyl-histone H3 was purchased from Millipore (Billerica, MA, USA). Anti-CCL20-neutralizing antibodies and mouse IgG isotype control were purchased from R&D Systems (Minneapolis, MN, USA). Phycoerythrin-conjugated anti-human CD196 (CCR6) antibody and its isotype control were purchased from Biolegend (San Diego, CA, USA).
The human intestinal epithelial cell lines Caco-2 and HT-29 were cultured in Dulbecco’s modified Eagle medium (Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Burlington, ON, Canada), 100 U/ml penicillin, and 100 µg/ml streptomycin (Hyclone). Cells were grown at 37°C in a 5% CO2-humidified incubator. Polarized Caco-2 cells were prepared as described previously (25). The human intestinal epithelial cell line SNU-407 was purchased from the Korean Cell Line Bank (Seoul, Korea) and maintained in Roswell Park Memorial Institute 1640 medium (Hyclone) containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a 5% CO2-humidified incubator.
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Complementary DNA (cDNA) was synthesized using random hexamers (Roche, Basel, Switzerland) and M-MLV reverse transcriptase (Promega, Madison, WI, USA). Amplification of cDNA was performed by PCR in a total volume of 20 µl containing EmeraldAmp PCR Master Mix (Takara Biomedical Inc., Osaka, Japan) and 10 pmol of primers specific for human chemokines or β-actin. The PCR conditions to amplify all chemokine genes used in this study were initial denaturation at 95°C for 5 min; amplification through 32 or 35 cycles of 95°C for 40 s, 60°C for 40 s, and 72°C for 40 s; and final extension at 72°C for 7 min. The PCR conditions for β-actin amplification were initial denaturation at 95°C for 5 min; amplification by 24 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 3 min; and a final extension at 72°C for 10 min. Real-time RT-PCR was performed with an Applied Biosystems 7500 real-time PCR system (Applied Biosystems, Waltham, MA, USA) as described previously (26). The sequences of the human chemokine-specific primers were as follows: CCL2, forward 5′-TCC CCA GAC ACC CTG TTT TA-3′ and reverse 5′-CAA AAC ATC CCA GGG GTA GA-3′; CCL5, forward 5′-GAA AGA ACC GCC AAG TGT GT-3′ and reverse 5′-GTA GAA TCT GGG CCC TTC AA-3′; CCL20, forward 5′-GCC AAT GAA GGC TGT GAC AT-3′ and reverse 5′-AAC CCC AGC AAG GTT CTT TC-3′; CXCL10, forward 5′-GAT GTT CTG ACC CTG CTT CA-3′ and reverse 5′-GAA AGA ATT TGG GCC CCT TG-3′ and; CCL25, forward 5′- GTC CAC ACC CAA GGT GTC TT-3′ and reverse 5′-TGT AGG GCG ACG GTT TTA TC-3′ and; CCL28, forward 5′-GCT GAT GGG GAT TGT GAC TT-3′ and reverse 5′-GTT TCG TGT TTC CCC TGA TG-3′ and; β-actin, forward 5′-GTG GGG CGC CCC AGG CAC CA-3′ and reverse 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′; GAPDH, forward 5′-AAG GTG AAG GTC GGA GTC AA-3′ and reverse 5′-ATG ACA AGC TTC CCG TTC TC-3′.
Caco-2 cells or other human intestinal epithelial cells were stimulated with the indicated stimuli, the cell culture supernatants were collected, and their chemokine contents were determined using the appropriate ELISA kit (R&D or Biolegend) according to the manufacturer’s instructions.
Caco-2 cells (4 × 105 cells/ml) were treated with Shanchol™ and/or butyrate for 2 h. The concentrations of extracellular ATP in the cell culture supernatants were determined with an ENLITIN® ATP Assay System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions.
Caco-2 cells (4 × 105 cells/ml) were treated with the indicated stimuli for 3 h. The cell lysates were prepared and Western blotting was performed as described previously (27, 28).
All experiments using human blood were performed after receiving approval from the Institutional Review Board of Seoul National University. Human blood was provided by the Korean Red Cross and all donors provided informed consent, and it was properly handled according to the standard operating procedure for biohazards recommended by the institutional biosafety committee. It has been demonstrated that human blood monocyte-derived DCs upregulated CCR6, which is known to interact with CCL20 (29). To investigate whether CCL20 derived from the co-treatment with Shanchol™ and butyrate, peripheral blood mononuclear cells were isolated by density-gradient centrifugation as described previously (30). For differentiation into immature DCs, purified CD14+ monocytes (2 × 106 cells/ml)were cultured in the presence of 10 ng/ml GM-CSF and 10 ng/ml IL-4 for 6 days. Immature DCs (1 × 106 cells/ml) were added to the upper chamber of a 24-well Transwell® support with a 5 µm pore polycarbonate membrane insert (Costar, Corning, NY, USA). Caco-2 cells (4 × 105 cells/ml) were stimulated with Shanchol™ and/or butyrate for 24 h, and then the culture supernatants (600 µl) were collected and moved to the lower chamber of the transwell plate. The cells and supernatants were incubated together at 37°C for 1.5 h. The migrated DCs in the lower chamber were counted using trypan blue staining. For neutralization, culture supernatants were incubated for 30 min at 37°C with 5 µg/ml of anti-CCL20-neutralizing antibody or its isotype control antibody.
Caco-2 cells (4 × 105 cells/ml) were treated with Shanchol™ and/or butyrate for 2 h. The acetylated histone H3 of the CCL20 promoter was determined by using ChIP assay as described previously (31). The cross-linked chromatin DNA was incubated at 4°C overnight with anti-acetylated histone H3 antibodies or its isotype control. The immunoprecipitated DNA was analyzed by real-time RT-PCR using primers specific to the CCL20 promoter (forward 5′-CTT TTC TGG GTC ACA GGG CT-3′ and reverse 5′-GTA CAC AGA AGG CGT GTT GC-3′).
Caco-2 cells (5 × 105 cells/ml) were plated for 6 h before transfection. The cells were then transfected overnight with pNF-κB-Luc or pAP-1-Luc (Clontech, Mountain View, CA, USA) using Attractene (Promega). Then, the cells were plated and treated with Shanchol™ and/or butyrate for 16 h. For reporter gene assays, the cells were lysed with Glo Lysis Buffer (Promega) and luciferase activity was then quantified using the Bright-Glo Luciferase Assay System (Promega) with a Spark™ 10-M multimode microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
All data are expressed as mean value ± SD of triplicates unless otherwise stated. Treatment groups were compared with an appropriate control group. Statistical significance was assessed using ANOVA performed in GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Differences were considered significant when P < 0.05.
First, we examined whether Shanchol™ induces chemokine expression in human intestinal epithelial cells. Shanchol™ treatment (106–109 CFU/ml) induced mRNA expression of CCL2, CCL5, CCL20, and CXCL10 in a dose-dependent manner in Caco-2 cells (Figure 1A and Figure S5 in Supplementary Material). Time course analysis showed that Shanchol™-induced mRNA expression of all chemokines tested peaked at 3–6 h after the treatment, with the exception of CCL5 (Figure 1B and Figure S5 in Supplementary Material). However, the mRNA expression levels of CCL3, CCL4, CCL25, and CCL28 were not altered by Shanchol™ treatment (data not shown). Interestingly, in contrast to the mRNA results, Shanchol™ did not induce secretion of CCL2, CCL5, CCL20, and CXCL10 from Caco-2 cells, though a little induction could be seen at 109 CFU/ml (Figure 1C). Similar results were also observed in HT-29 cells (Figure S1A–C in Supplementary Material). These results indicate that Shanchol™ treatment potently induces mRNA synthesis of chemokines but barely induces protein secretion from human intestinal epithelial cells.
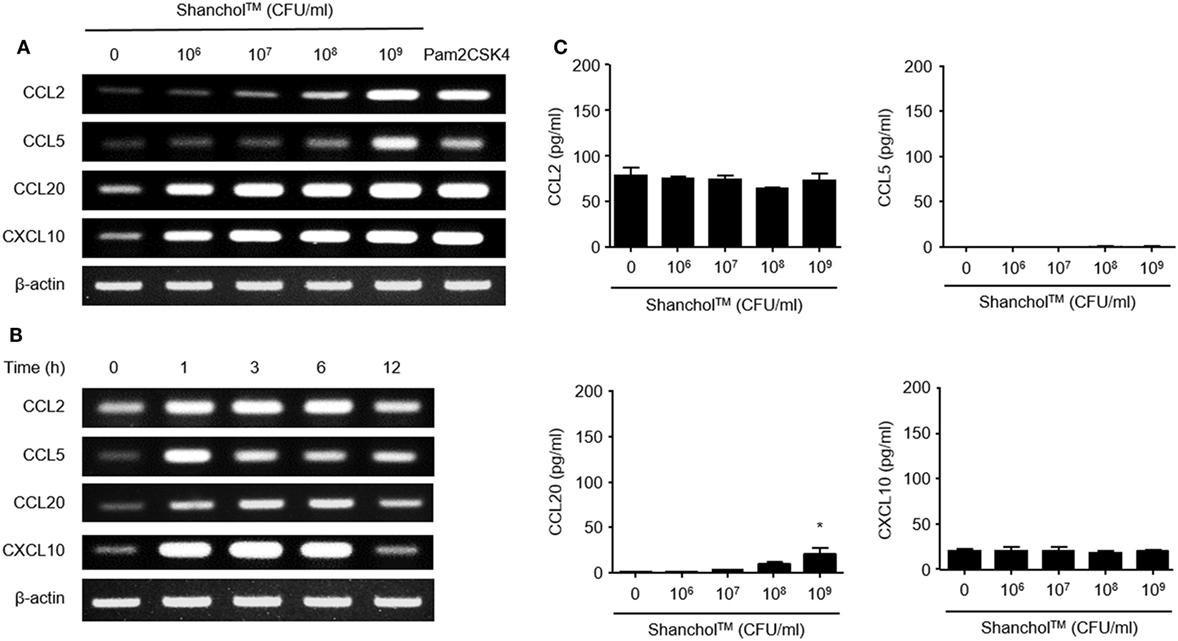
Figure 1. Shanchol™ induces chemokine mRNA expression but hardly induces chemokine secretion in Caco-2 cells. Caco-2 cells were stimulated (A) with various concentration of Shanchol™ (106–109 CFU/ml) or Pam2CSK4 (1 µg/ml) for 3 h, or (B) with Shanchol™ (108 CFU/ml) for various time periods. Total RNA was extracted and the mRNA expression levels of CCL2, CCL5, CCL20, and CXCL10 were determined by RT-PCR. Data shown are representative of three independent experiments. (C) Caco-2 cells were treated with various concentration of Shanchol™ (106–109 CFU/ml) for 24 h. Then, the cell culture supernatants were collected, and the concentrations of CCL2, CCL5, CCL20, and CXCL10 were determined by ELISA. Data shown are representative of three independent experiments. All results are expressed as mean ± SD of triplicate samples. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with control.
Microbiota-associated metabolites such as SCFAs have been reported to play a role in immunomodulation in the gut (32). To examine whether SCFAs alter chemokine secretion, Caco-2 cells were treated with Shanchol™ in the presence of major intestinal SCFAs such as acetate, butyrate, and propionate for 24 h, and chemokine secretion was determined by using ELISA. Interestingly, only butyrate enhanced CCL20 secretion in the presence of Shanchol™. However, neither acetate nor propionate enhanced CCL20 secretion from Caco-2 cells. Remarkably, the secretion of CCL2, CCL5, or CXCL10 was not altered even in the presence of butyrate (Figure 2A). Shanchol™ dose-dependently induced CCL20 secretion in the presence of butyrate (Figure 2B), and butyrate potently increased Shanchol™-induced CCL20 production in a dose-dependent manner (Figure 2C). Such cooperative effect of Shanchol™ and butyrate was observed in the CCL20 mRNA expression, but not in the mRNA expression of other chemokines, including CCL2, CCL5, CXCL10, CCL25, or CCL28 (Figure S2 in Supplementary Material). Furthermore, Shanchol™-induced secretion of CCL20, but not CXCL10, was facilitated by the presence of butyrate in SNU-407 human intestinal epithelial cells (Figure 2D). Caco-2 cells have been reported to differentiate and polarize into cells with intestinal enterocyte-like features (28). Therefore, to mimic the human intestinal epithelium, Caco-2 cells were cultured for 4 weeks on a transwell-permeable filter until the cells were fully differentiated and polarized. As shown in Figure 2E, CCL20 secretion was enhanced in both apical and basolateral compartments of polarized Caco-2 cells, regardless of whether the Shanchol™ and butyrate co-treatment was apical or basolateral.
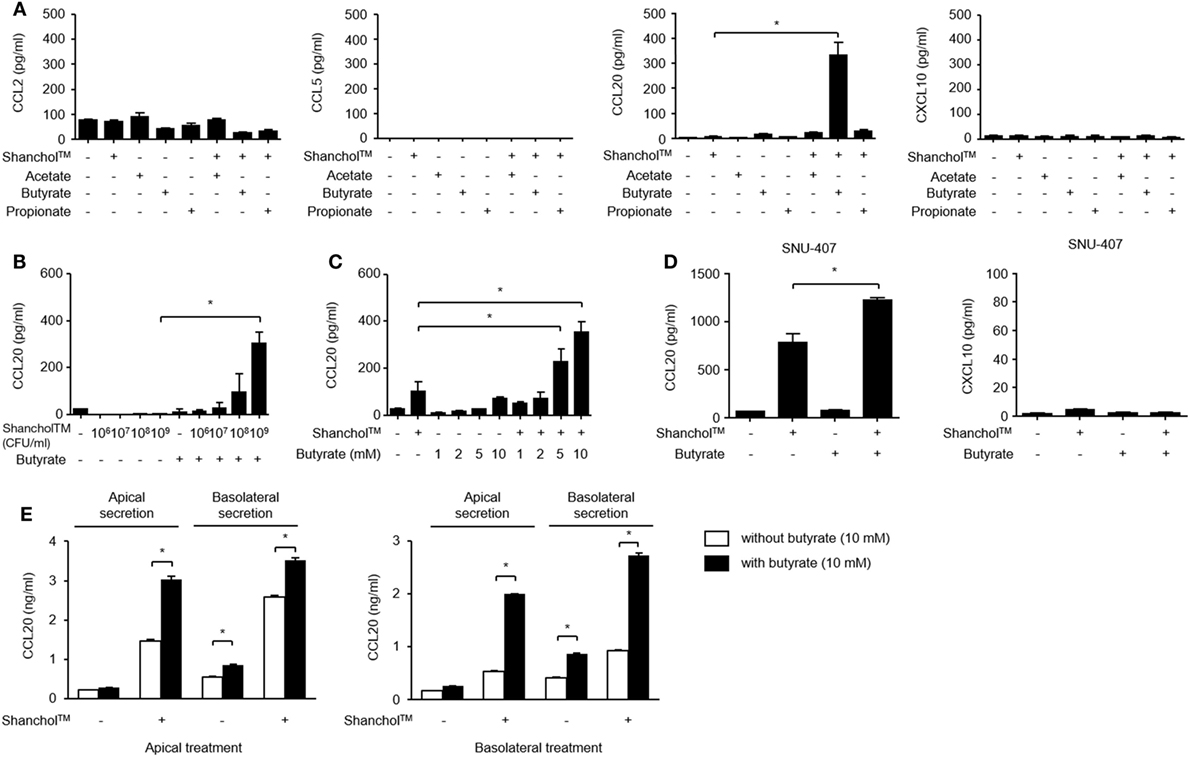
Figure 2. Shanchol™ significantly induces CCL20 secretion in the presence of butyrate in Caco-2 cells. (A) Caco-2 cells were stimulated with Shanchol™ (109 CFU/ml) in the presence or absence of acetate, butyrate, or propionate (10 mM). (B,C) Caco-2 cells were incubated with various concentrations of Shanchol™ (106–109 CFU/ml) in the presence of butyrate (10 mM) (B) or with various concentrations of butyrate (1–10 mM) in the presence of Shanchol™ (109 CFU/ml) (C) for 24 h. (D) SNU-407 cells were stimulated with Shanchol™ (109 CFU/ml) in the presence or absence of 10 mM butyrate for 24 h. (E) Caco-2 cells were plated on Transwell® supports for 4 weeks and then apically or basolaterally treated with Shanchol™ (109 CFU/ml) and/or butyrate (10 mM). The culture supernatants in the apical and basolateral compartments were then collected. The concentrations of secreted CCL2, CCL5, CCL20, and CXCL10 were measured in the culture supernatants by ELISA. N.D., not detected. All results are expressed as mean ± SD of triplicate samples. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with the appropriate control.
GPR43 and GPR109A are butyrate receptors that regulate various immune responses such as leukocyte migration and lymphocyte activation (33). It has been reported that Gram-negative bacteria and their lipopolysaccharide (LPS) induced GPR43 and GPR109A expression (34, 35). To determine whether GPR43 or GPR109A is involved in butyrate-mediated stimulation of CCL20 secretion, we examined GPR43 and GPR109A expression in Caco-2 cells. In Caco-2 cells treated with Shanchol™, mRNA expression of both GPR43 and GPR109A was induced and butyrate augmented only GPR109A mRNA expression but not GPR43 mRNA expression (Figure 3A and Figure S5 in Supplementary Material). We next examined whether GPR109A is involved in CCL20 secretion. When Caco-2 cells were pretreated with a specific inhibitor of GPR109A (MPN) or a GPCR inhibitor (pertussis toxin) for 1 h and then treated with Shanchol™ and/or butyrate for an additional 24 h, CCL20 secretion was significantly suppressed by both GPR109A inhibitor and GPCR inhibitor (Figures 3B,C). Since the activation of GPR109A is associated with the downstream signaling mediators, such as mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and reactive oxygen species (ROS) (36–38), we investigated the enhancement of CCL20 production in Caco-2 cells pretreated with the specific inhibitors for MAPK, PKC, or ROS. CCL20 secretion was remarkably inhibited by MAPK-specific inhibitors (SB203580 for p38 kinase, PD98059 for ERK, and SP600125 for JNK) (Figure 3D). Furthermore, CCL20 secretion was downregulated by pretreatment with inhibitors for ROS or PKC (Figure 3E), suggesting that GPR109A, MAPK, PKC, and ROS signaling mediate the enhancement of CCL20 secretion in Caco-2 cells.
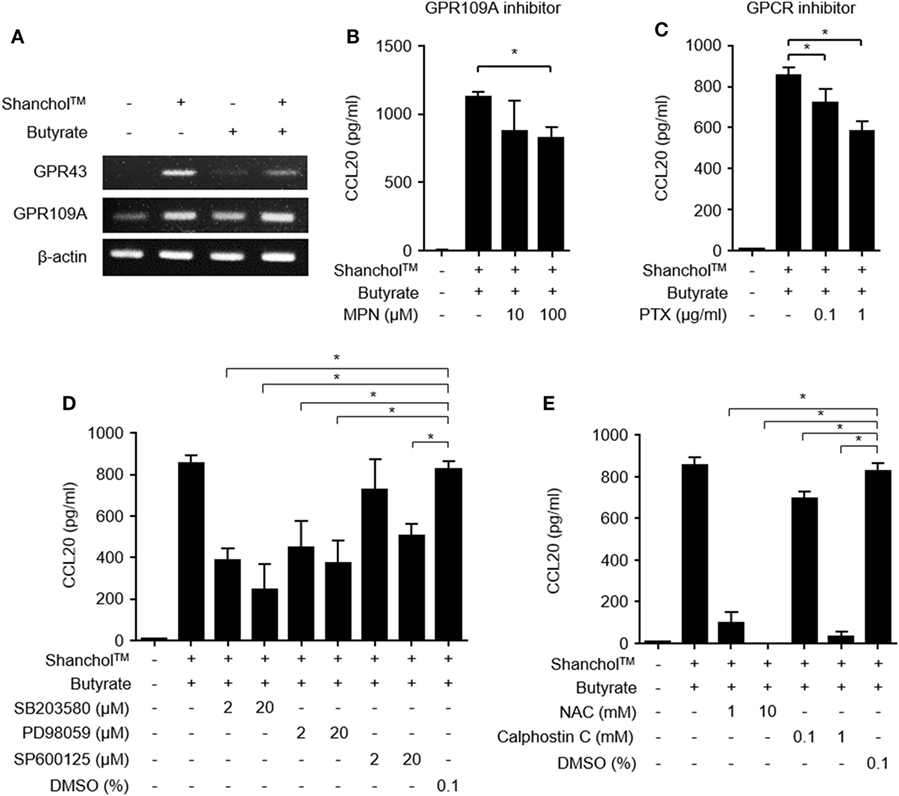
Figure 3. Activation of GPR109A and MAPK is involved in CCL20 secretion from Caco-2 cells. (A) Caco-2 cells were stimulated with Shanchol™ (109 CFU/ml) in the presence or absence of butyrate (10 mM) for 3 h. Total RNA was extracted, after which the mRNA expression levels of GPR43 and GPR109A were determined using RT-PCR. (B–E) Caco-2 cells were pretreated with the indicated concentrations of mepenzolate bromide (MPN) (B), pertussis toxin (PTX) (C), SB203580, PD98059, SP600125, or DMSO (vehicle control) (D), and NAC, Calphostin C, or DMSO (vehicle control) (E) for 1 h. Then, cells were co-stimulated with Shanchol™ (109 CFU/ml) and butyrate (10 mM) for an additional 24 h, the culture supernatants were collected, and the concentrations of secreted CCL20 were measured by ELISA. One of three representative results is shown. All results are expressed as mean ± SD of triplicate samples. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with the appropriate control.
Butyrate is a source of metabolic energy (i.e., ATP) in colonocytes (39), where ATP enhances IL-6 and KC chemokine production in murine intestinal epithelial cells (40). To investigate whether butyrate induces ATP secretion in Caco-2 cells, the extracellular concentrations of ATP were determined in the presence or absence of the Shanchol™/butyrate. As shown in Figure 4A, extracellular ATP production was increased in Caco-2 cells co-treated with Shanchol™ and butyrate. Blocking the interaction between ATP and P2X7 receptor by treatment with a P2X7 purinergic receptor antagonist (oxATP) dramatically attenuated CCL20 secretion (Figure 4B). Moreover, exogenously treated ATP augmented CCL20 mRNA expression and secretion in the presence of Shanchol™ (Figures 4C,D, respectively), whereas addition of exogenous ATP did not affect Shanchol™-induced CXCL10 mRNA expression or secretion (Figure S3 in Supplementary Material). These results suggest that butyrate-induced ATP secretion is crucial for the enhancement of Shanchol™-induced CCL20 production. However, pretreatment with a caspase-1 or caspase-4 inhibitor did not suppress CCL20 secretion (Figures 4E,F, respectively). Thus, those results suggest that ATP-P2X7 signaling, but not inflammasome activation, is essential for the synergistic production of CCL20 in response to ShancholTM and butyrate.
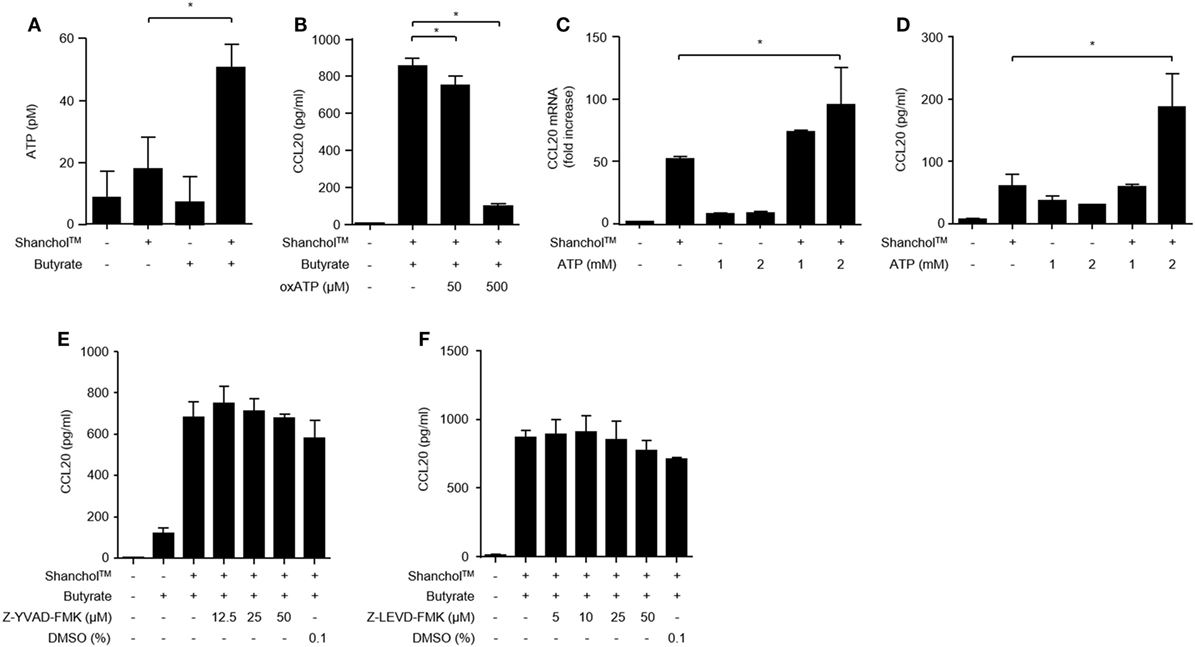
Figure 4. ATP is required for CCL20 secretion from Caco-2 cells. (A) Caco-2 cells were incubated with Shanchol™ in the presence or absence of 10 mM butyrate for 2 h. Then, the culture supernatants were collected, and extracellular ATP production was measured. (B) Caco-2 cells were pretreated with 50 or 500 µM of oxATP for 1 h and subsequently stimulated with Shanchol™ and butyrate for an additional 24 h. Then, the culture supernatants were collected, and the concentrations of secreted CCL20 were measured by ELISA. Data shown are representative of three independent experiments. (C) Caco-2 cells were stimulated with the indicated concentration of ATP in the presence or absence of Shanchol™ for 3 h. Total RNA was isolated, and the mRNA expression level of CCL20 was determined by real-time RT-PCR. (D) Caco-2 cells were stimulated with the indicated concentration of ATP in the presence or absence of Shanchol™ for 24 h. Then, the culture supernatants were collected, and the secreted CCL20 was measured by ELISA. (E,F) Caco-2 cells were pretreated with the indicated concentrations of the caspase-1 inhibitor (Z-YVAD-FMK) (E), the caspase-4 inhibitor (Z-LEVD-FMK) (F), or DMSO (vehicle control) for 1 h and subsequently stimulated with Shanchol™ and butyrate for an additional 24 h. Then, the culture supernatants were collected and the CCL20 secretion was measured by ELISA. Data shown are representative of three independent experiments. All results are expressed as mean ± SD of triplicate samples. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with the appropriate control.
Butyrate has been reported to act as an HDAC inhibitor (41) and regulates the transcription of chemokines and antimicrobial peptides in epithelial cells (42). Therefore, Caco-2 cells were treated with Shanchol™ in the presence of TSA, an HDAC inhibitor. CCL20 mRNA expression was increased by co-treatment with Shanchol™ and TSA, similar to the results for co-treatment with Shanchol™ and butyrate. However, TSA did not enhance Shanchol™-induced CXCL10 mRNA expression as butyrate did not (Figure 5A). Besides, co-treatment with Shanchol™ plus butyrate or Shanchol™ plus TSA resulted in increased CCL20 secretion, but not increased CXCL10 secretion (Figure 5B). The CCL20 promoter region contains binding sites for the transcription factors NF-κB and AP-1. Reporter gene assay showed that co-treatment with Shanchol™ and butyrate synergistically induced AP-1 activation, while butyrate alone or together with Shanchol™ substantially increased NF-κB activation (Figure 5C). Increased acetylation of histone H3 lysine residues was observed when cells were co-treated with butyrate or TSA (Figure 5D and Figure S6 in Supplementary Material). ChIP assay showed that butyrate augmented acetylation of histone H3 in the CCL20 promoter region though co-treatment with Shanchol™ and butyrate did not enhance histone acetylation (Figure 5E and Figure S7 in Supplementary Material). Taken together, these results suggest that HDAC inhibition by butyrate is involved in synergistic CCL20 production at the transcriptional level in Caco-2 cells.
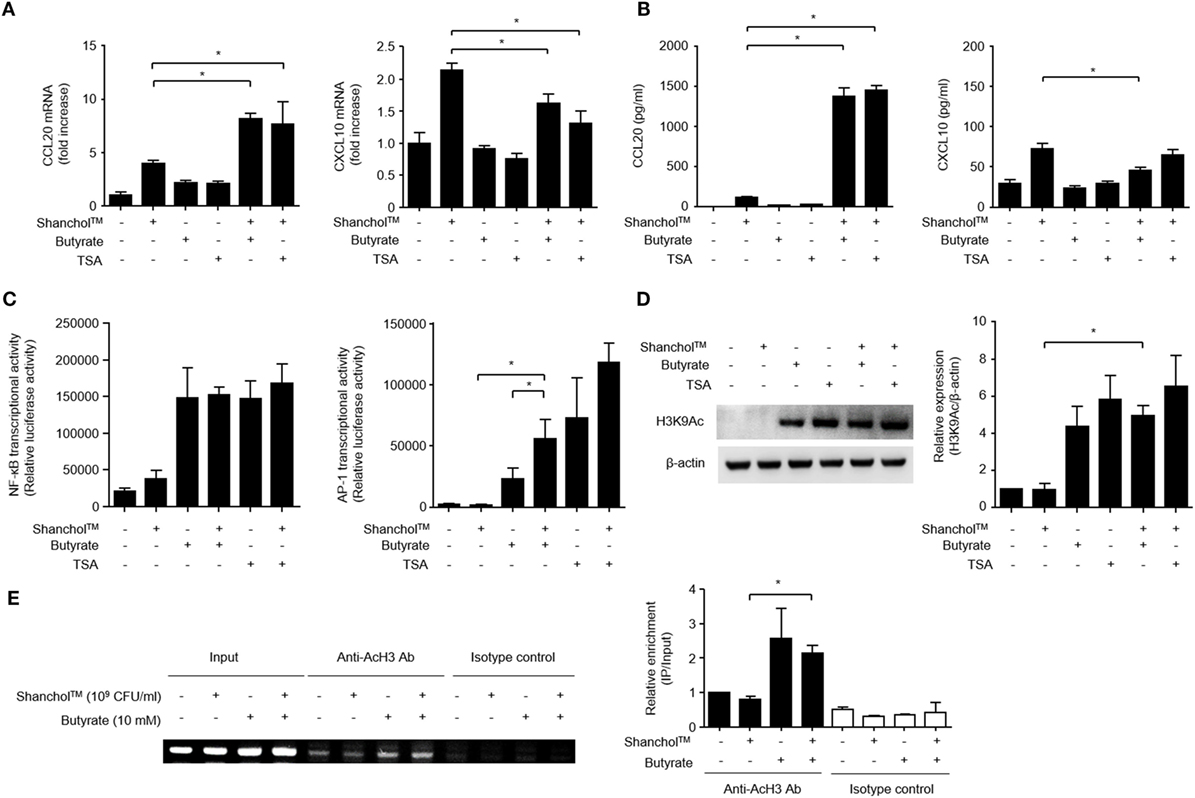
Figure 5. Histone deacetylase inhibition is necessary for CCL20 production in Caco-2 cells. (A) Caco-2 cells were stimulated with Shanchol™ in the presence of 10 mM butyrate or 5 µM trichostatin A (TSA) for 3 h. Total RNA was isolated, and the mRNA expression level of CCL20 or CXCL10 was determined by real-time RT-PCR. (B) Caco-2 cells were treated with Shanchol™ in the presence of 10 mM butyrate or 5 µM TSA for 24 h. Then, the culture supernatants were collected, and the concentrations of CCL20 and CXCL10 were determined by ELISA. All results are expressed as mean ± SD of triplicate samples. (C) Caco-2 cells transfected with a pNF-κB-luc or pAP-1uc luciferase plasmid were further treated with Shanchol™ in the presence of butyrate or TSA for 16 h. Then, the cells were lysed, and subjected to the luciferase assay. (D) Caco-2 cells were stimulated with Shanchol™ in the presence of butyrate or TSA for 3 h. Cell lysates were generated and subjected to Western blot analysis to examine histone acetylation at lysine residues. The relative ratio of acetylated-H3K9 to β-actin was obtained by densitometry. (E) Caco-2 cells were treated with Shanchol™ and/or butyrate for 2 h. Then, the cells were subjected to ChIP assay. Cross-linked chromatin extracts were immunoprecipitated with anti-acetylated histone H3 antibodies (Anti-AcH3 Ab) or rabbit IgG antibodies (Isotype control). DNA fragments were analyzed by PCR (left) and by real-time RT-PCR (right) using primers specific to the human CCL20 promoter. The results shown are representative of three independent experiments. Data are presented as mean ± SD of three independent experiments. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with the appropriate control.
CCL20 has been shown to promote the migration of immature DCs (43). Thus, we assessed the migratory capacity of human immature DCs in response to Shanchol™ and butyrate. Human immature DCs expressed CCR6 which is the receptor for CCL20 (Figure S4 in Supplementary Material). Significantly more DC migration was observed in response to co-treatment with Shanchol™ and butyrate compared with the level in response to either Shanchol™ or butyrate alone (Figure 6), suggesting that co-treatment with Shanchol™ and butyrate-induced CCL20 might be associated with the augmented DC migration. In addition, the migration of DCs was inhibited by incubation with CCL20-neutralizing antibodies indicating that this migration specifically depends on CCL20-CCR6 chemotaxis.
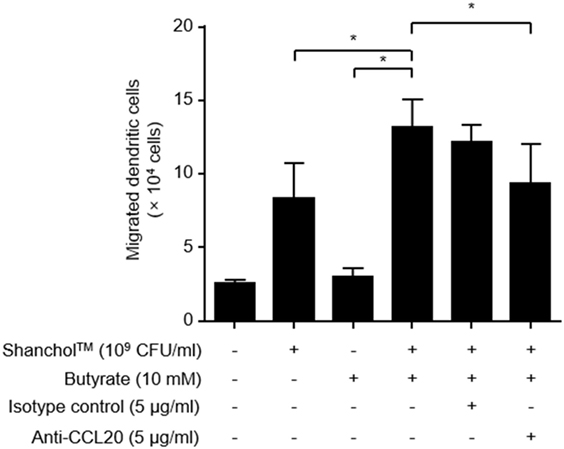
Figure 6. Co-treatment with Shanchol™ and butyrate induces chemotactic migration of human immature dendritic cells (DCs). Caco-2 cells were stimulated with Shanchol™ (109 CFU/ml) in the presence or absence of butyrate (10 mM) for 24 h. After that, the culture supernatants were pre-incubated with indicated concentration of anti-CCL20-neutralizing antibody or isotype control for 30 min. Immature DCs were placed in the upper chamber and further incubated for 1.5 h. The migrated DCs in the lower chamber were counted using trypan blue staining. All results are expressed as mean ± SD of triplicate samples. The asterisk (*) indicates a statistically significant difference (P < 0.05) compared with the appropriate control.
SCFAs are known to modulate the host immune responses by binding to receptors such as GPR43 and GPR109A or through HDAC inhibition (19). Butyrate is an SCFA that has been shown to stimulate GPR109A and enhance IL-18 production in the intestinal epithelium (44). However, the mechanisms by which SCFAs affect vaccine-induced chemokine expression in intestinal epithelial cells have not yet been elucidated. In the present study, we showed that the killed whole-cell oral cholera vaccine Shanchol™ potently induced chemokine mRNA expression in human intestinal epithelial cells, whereas it barely induced chemokine protein secretion. Consistent with our results, IL-8 mRNA expression has been shown to be substantially induced in HT-29-18N2 cells in response to cholera vaccine strains, whereas negligible protein secretion was observed (<50 pg/ml) (45). Notably, CCL20 was reported to be expressed at both mRNA and protein levels in follicle-associated epithelium of Peyer’s patches but not in the intestinal villus epithelium in mice (46). This discrepancy might be attributed to species difference, because CCL20 was weakly expressed in normal human colon epithelium and its expression was substantially increased in response to inflammatory cytokines (47). In addition, the present study demonstrated that butyrate potently enhanced Shanchol™-induced expression of CCL20 in human intestinal epithelial cells, but not any other chemokines tested. Our studies and others for elucidating the relevant molecular mechanisms have demonstrated that epigenetic modification (48), ATP-P2X7 signaling (49) and GPR109A-mediated pathways are associated with butyrate-mediated enhancement of CCL20 production, as summarized in Figure 7. We also showed that migration of human immature DCs was increased in the presence of Shanchol™ and butyrate, implying that butyrate contributes to the enhancement of mucosal immune responses induced by oral cholera vaccines in the gut.
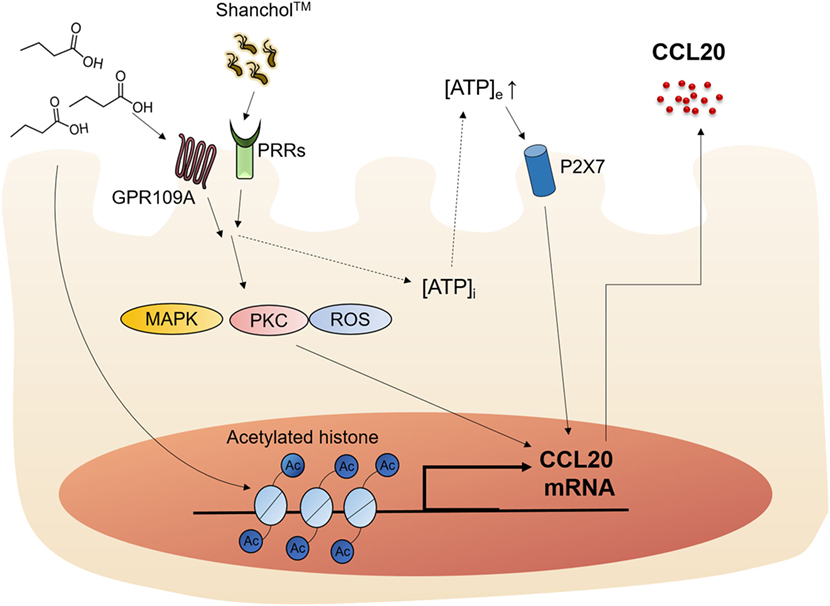
Figure 7. Schematic illustration of the proposed action mechanism. Butyrate enhances Shanchol™-induced CCL20 secretion via GPR109A, ATP-P2X7, and epigenetic control by histone deacetylase inhibition.
Gram-negative bacteria contain diverse microbe-associated molecular patterns, such as LPS and lipoproteins, which are generally recognized by toll-like receptor 4 (TLR4) and TLR2, respectively (50). Although Shanchol™ comprises Gram-negative bacteria, V. cholerae strains, our group showed that V. cholerae preferentially induced TLR2 activation, but not TLR4 activation, ultimately resulting in pro-inflammatory responses (51). Furthermore, a porin protein of V. cholerae, OmpU, exclusively interacted with TLR2, and induced IL-8 production (52), while LPS of V. cholerae failed to induce IL-8 production in HT-29 cells (9). Although further comprehensive studies are needed, OmpU of V. cholerae would be one of the major components to induce chemokine production in human intestinal epithelial cells.
SCFAs are saturated fatty acids consisting of one to six carbons; acetate (C2), propionate (C3), and butyrate (C4). The carbon chain length of SCFAs has been proposed to affect their immunomodulatory potencies (53). In support of this idea, acetate, propionate, and butyrate all activate GPCRs, whereas GPR109A is recognized only by butyrate but not by acetate or propionate (18). In addition, chain length of SCFAs affects the ability to stimulate chemokine production by altering histone acetylation. SCFAs with longer carbon chain lengths are known to be more potent inducers of histone acetylation (53). For example, butyrate has more potent histone-acetylating activity by interfering with HDAC compared with acetate and propionate (42). In this study, we demonstrated that only butyrate substantially augments CCL20 production in Caco-2 cells. Furthermore, we also found that the GPR109A-mediated pathways and HDAC inhibition are associated with enhancement of CCL20 production by butyrate. Thus, the chain length of SCFAs might be associated with the potency of the SCFA to activate GPCRs and regulate epigenetic modification, consequently resulting in the modulation of chemokine expression by butyrate.
Increased level of intracellular ATP was found in Caco-2 cells in response to butyrate (54). However, bacterial components including TLR ligands promoted extracellular ATP production (55). Indeed, we found that simultaneous treatment with Shanchol™ and butyrate significantly increased extracellular ATP production, while butyrate alone could not increase extracellular ATP level. Furthermore, supernatants from butyrate-treated Caco-2 cells without Shanchol™ treatment did not considerably induce CCL20 production (Figure S8 in Supplementary Material), suggesting that Shanchol™ stimulates the excretion of ATP to induce CCL20 production.
In this study, we demonstrated that ATP-P2X7 signaling plays a key role in CCL20 production in Caco-2 cells. This finding is consistent with previous studies reporting that high levels of ATP were released to the extracellular space, and that purinergic receptors such as P2X7 were activated in epithelial cells upon exposure to mechanical stress, hypotonic media, vasoactive agents, or inflammatory stimuli (56). This release of ATP resulted in the release of CCL2, CCL5, and CXCL8, while it inhibited the expression of CXCL10 via P2X7 and P2Y1 receptor signaling (57). Activation of the P2X7 receptor by extracellular ATP induces Ca2+ influx and K+ efflux, thereby activating the NLRP3 inflammasome (58). However, pretreatment with caspase-1 or caspase-4 inhibitors did not affect CCL20 production in this study, suggesting that inflammasome activation is not involved in CCL20 secretion. In addition to activating the inflammasome, P2X7 receptor activation increases the intracellular Ca2+ concentration, thereby activating downstream signaling pathways such as the MAPK p38 pathway (59). However, our observation indicates that an intracellular Ca2+ chelating regent, BAPTA-AM, did not abrogate CCL20 production (Figure S9 in Supplementary Material), suggesting that Ca2+ influx is not involved in the enhancement of CCL20 secretion. Although further studies are needed, butyrate-mediated GPR109A and ATP-P2X7 may induce intracellular signaling pathways such as MAPK pathway, leading to the enhancement of CCL20 secretion.
Epigenetic control is a phenomenon by which gene expression and function are regulated in various cell types without changing the nucleotide sequence. This type of control is mediated by histone modification and DNA methylation. Histone acetylation is catalyzed by histone acetyltransferases, and is related to transcriptional activation by allowing the transcription machinery to access the DNA binding sites. We found that HDAC inhibition by butyrate or TSA induced histone acetylation at lysine residues and strongly increased Shanchol™-induced CCL20 mRNA and protein production in Caco-2 cells. In accordance with our observation, butyrate has been shown to induce CCL20 production on the transcriptional level via HDAC inhibition in gingival epithelial cells (48) and in intestinal epithelial cells (60). However, we also found that butyrate and TSA synergistically increased AP-1 and downregulated the expression of other chemokines (e.g., CCL2 and CXCL10). These findings are consistent with previous reports that butyrate or TSA enhances PMA-induced AP-1 response in human intestinal cells (61), and AP-1 negatively regulated CXCL10 induction in response to TLR3 or RIG-1 ligand in hepatocytes (62). In addition, it has been demonstrated that HDAC inhibition enhances TLR-induced TNF-α expression in HT-29 cells, whereas it blocks IL-8 and MCP-1 expression (63). Therefore, it is likely that HDAC inhibition alone cannot upregulate the expression of all chemokines on the transcriptional level.
Chemokine production is important for the induction of mucosal immune responses following vaccination. Although many chemokines are associated with inflammatory responses, recent researches in the biological roles of chemokines such as CCL20 also allow to employ as potential vaccine adjuvants by regulating mucosal immune responses (16, 43). Studies have shown that mucosal vaccination increases local chemokine production in the gut mucosa; these chemokines attract immune cells (including IgA-producing plasma cells) to the site of vaccination (64, 65). In fact, chemokines such as CCL20 have been used as adjuvants for several cancer vaccines and for DNA vaccines against simian immunodeficiency virus infection (66–68). CCR6, the CCL20 receptor, is important for the recruitment of DCs, memory T cells, and B cells, which are responsible for protective immunity. Several studies have shown that gut microbiota is closely associated with vaccine effectiveness (69, 70). The treatment with prebiotics such as dietary fibers promotes the production of SCFAs in the gut that increases the immunogenicity and efficacy of vaccines (71, 72). In addition, the administration with Bifidobacterium, which is a major bacterium producing SCFAs in the intestine, evidently increased the level of serum and fecal IgA (73), suggesting that the SCFA such as butyrate could enhance the effectiveness of cholera vaccine. We found that co-treatment with Shanchol™ and butyrate potently induced CCL20 secretion. Since this cytokine is associated with the migration of immature DCs, our results suggest that butyrate accelerates Shanchol™-induced mucosal immune responses in the gut. Since CCL20 induces chemotaxis of immune cells that impact vaccine efficacy, our findings indicate that butyrate could be used to enhance mucosal vaccine-induced immune responses. Although further studies are needed to establish that the use of butyrate correlates with the induction of protective immunity, the finding that butyrate regulates intestinal immune responses when used together with an oral cholera vaccine provides an important first step toward achieving enhanced mucosal vaccine efficacy in the gut.
This study was carried out in accordance with the recommendations of Institutional Review Board with written informed consent from all subjects and with the Declaration of Helsinki.
SH conceived the idea. J-RS, S-SK, and SH designed the experiments. J-RS and S-SK performed experiments. J-RS, S-SK, and SH analyzed and/or interpreted the data and contributed to the discussion of the results followed by writing and reviewing the manuscript. DL and C-HY provided critical comments and contributed to the discussion of the results followed by writing and reviewing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by grants from the Agency for Defense Development (UD150030ID), the National Research Foundation of Korea (NRF), funded by the Korean government (NRF-2015M2A2A6A01044894) and the Next-Generation BioGreen 21 Program (PJ01112402), Rural Development Administration, Republic of Korea.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fimmu.2018.00055/full#supplementary-material.
CTB, cholera toxin B subunit; GALT, gut-associated lymphoid tissue; APC, antigen-presenting cell; DC, dendritic cell; SCFA, short-chain fatty acid; HDAC, histone deacetylase; GPCR, G-protein-coupled receptor; ATP, adenosine triphosphate; TSA, trichostatin A; MPN, mepenzolate bromide; PTX, pertussis toxin; ROS, reactive oxygen species; PKC, protein kinase C; HAT, histone acetyltransferase.
1. Rabbani GH, Greenough WB III. Food as a vehicle of transmission of cholera. J Diarrhoeal Dis Res (1999) 17(1):1–9.
2. Holmgren J, Svennerholm AM, Jertborn M, Clemens J, Sack DA, Salenstedt R, et al. An oral B subunit: whole cell vaccine against cholera. Vaccine (1992) 10(13):911–4. doi:10.1016/0264-410X(92)90324-D
3. Kabir S. Critical analysis of compositions and protective efficacies of oral killed cholera vaccines. Clin Vaccine Immunol (2014) 21(9):1195–205. doi:10.1128/CVI.00378-14
4. Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis (2011) 5(10):e1289. doi:10.1371/journal.pntd.0001289
5. Yang JS, Kim HJ, Yun CH, Kang SS, Im J, Kim HS, et al. A semi-automated vibriocidal assay for improved measurement of cholera vaccine-induced immune responses. J Microbiol Methods (2007) 71(2):141–6. doi:10.1016/j.mimet.2007.08.009
6. Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J. Cholera. Lancet (2017) 390(10101):1539–49. doi:10.1016/S0140-6736(17)30559-7
7. Kang SS, Yang JS, Kim KW, Yun CH, Holmgren J, Czerkinsky C, et al. Anti-bacterial and anti-toxic immunity induced by a killed whole-cell-cholera toxin B subunit cholera vaccine is essential for protection against lethal bacterial infection in mouse pulmonary cholera model. Mucosal Immunol (2013) 6(4):826–37. doi:10.1038/mi.2012.121
8. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med (2005) 11(4 Suppl):S45–53. doi:10.1038/nm1213
9. Kang SS, Jeon JH, Woo SJ, Yang JS, Kim KW, Yun CH, et al. IFN-gamma renders human intestinal epithelial cells responsive to lipopolysaccharide of Vibrio cholerae by down-regulation of DMBT1. Comp Immunol Microbiol Infect Dis (2012) 35(4):345–54. doi:10.1016/j.cimid.2012.02.003
10. Campbell DJ, Butcher EC. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J Clin Invest (2002) 110(8):1079–81. doi:10.1172/JCI16946
11. Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol (2003) 170(3):1452–61. doi:10.4049/jimmunol.170.3.1452
12. Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem (1997) 272(23):14893–8. doi:10.1074/jbc.272.23.14893
13. Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med (1997) 186(6):837–44. doi:10.1084/jem.186.6.837
14. Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science (1998) 279(5349):381–4. doi:10.1126/science.279.5349.381
15. Krzysiek R, Lefevre EA, Bernard J, Foussat A, Galanaud P, Louache F, et al. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells. Blood (2000) 96(7):2338–45.
16. Kodama S, Abe N, Hirano T, Suzuki M. A single nasal dose of CCL20 chemokine induces dendritic cell recruitment and enhances nontypable Haemophilus influenzae-specific immune responses in the nasal mucosa. Acta Otolaryngol (2011) 131(9):989–96. doi:10.3109/00016489.2011.576429
17. Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A (2006) 103(24):9178–83. doi:10.1073/pnas.0602888103
18. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res (2009) 69(7):2826–32. doi:10.1158/0008-5472
19. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol (2015) 8(1):80–93. doi:10.1038/mi.2014.44
20. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem (2003) 278(13):11312–9. doi:10.1074/jbc.M211609200
21. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem (2011) 22(9):849–55. doi:10.1016/j.jnutbio.2010.07.009
22. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut (1987) 28(10):1221–7. doi:10.1136/gut.28.10.1221
23. Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe (2016) 20(2):202–14. doi:10.1016/j.chom.2016.07.001
24. Kim YG. Microbiota influences vaccine and mucosal adjuvant efficacy. Immune Netw (2017) 17(1):20–4. doi:10.4110/in.2017.17.1.20
25. Woo SJ, Im J, Jeon JH, Kang SS, Lee MH, Yun CH, et al. Induction of BAFF expression by IFN-gamma via JAK/STAT signaling pathways in human intestinal epithelial cells. J Leukoc Biol (2013) 93(3):363–8. doi:10.1189/jlb.0412210
26. Kang SS, Kim HJ, Jang MS, Moon S, In Lee S, Jeon JH, et al. Gene expression profile of human peripheral blood mononuclear cells induced by Staphylococcus aureus lipoteichoic acid. Int Immunopharmacol (2012) 13(4):454–60. doi:10.1016/j.intimp.2012.05.010
27. Kim AY, Kwak JH, Je NK, Lee YH, Jung YS. Epithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicol Res (2015) 31(2):151–6. doi:10.5487/TR.2015.31.2.151
28. Noh SY, Kang SS, Yun CH, Han SH. Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Mol Immunol (2015) 64(1):183–9. doi:10.1016/j.molimm.2014.11.014
29. Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, et al. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol (2000) 164(5):2285–95. doi:10.4049/jimmunol.164.5.2285
30. Kim SK, Yun CH, Han SH. Enhanced anti-cancer activity of human dendritic cells sensitized with gamma-irradiation-induced apoptotic colon cancer cells. Cancer Lett (2013) 335(2):278–88. doi:10.1016/j.canlet.2013.02.038
31. Kim NJ, Ahn KB, Jeon JH, Yun CH, Finlay BB, Han SH. Lipoprotein in the cell wall of Staphylococcus aureus is a major inducer of nitric oxide production in murine macrophages. Mol Immunol (2015) 65(1):17–24. doi:10.1016/j.molimm.2014.12.016
32. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature (2009) 461(7268):1282–6. doi:10.1038/nature08530
33. Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun (2003) 303(4):1047–52. doi:10.1016/S0006-291X(03)00488-1
34. Feingold KR, Moser A, Shigenaga JK, Grunfeld C. Inflammation stimulates niacin receptor (GPR109A/HCA2) expression in adipose tissue and macrophages. J Lipid Res (2014) 55(12):2501–8. doi:10.1194/jlr.M050955
35. Senga T, Iwamoto S, Yoshida T, Yokota T, Adachi K, Azuma E, et al. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood (2003) 101(3):1185–7. doi:10.1182/blood-2002-06-1881
36. Li G, Deng X, Wu C, Zhou Q, Chen L, Shi Y, et al. Distinct kinetic and spatial patterns of protein kinase C (PKC)- and epidermal growth factor receptor (EGFR)-dependent activation of extracellular signal-regulated kinases 1 and 2 by human nicotinic acid receptor GPR109A. J Biol Chem (2011) 286(36):31199–212. doi:10.1074/jbc.M111.241372
37. McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science (2000) 290(5496):1574–7. doi:10.1126/science.290.5496.1574
38. Chen L, So WY, Li SY, Cheng Q, Boucher BJ, Leung PS. Niacin-induced hyperglycemia is partially mediated via niacin receptor GPR109a in pancreatic islets. Mol Cell Endocrinol (2015) 404:56–66. doi:10.1016/j.mce.2015.01.029
39. Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab (2011) 13(5):517–26. doi:10.1016/j.cmet.2011.02.018
40. Yao Y, Levings MK, Steiner TS. ATP conditions intestinal epithelial cells to an inflammatory state that promotes components of DC maturation. Eur J Immunol (2012) 42(12):3310–21. doi:10.1002/eji.201142213
41. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A (2014) 111(6):2247–52. doi:10.1073/pnas.1322269111
42. Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr (2004) 134(9):2450S–4S.
43. Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity (2000) 12(5):495–503. doi:10.1016/S1074-7613(00)80201-0
44. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun (2015) 6:6734. doi:10.1038/ncomms7734
45. Rodriguez BL, Rojas A, Campos J, Ledon T, Valle E, Toledo W, et al. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect Immun (2001) 69(1):613–6. doi:10.1128/IAI.69.1.613-616.2001
46. Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J Immunol (2003) 171(6):2797–803. doi:10.4049/jimmunol.171.6.2797
47. Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol (2001) 280(4):G710–9. doi:10.1152/ajpgi.2001.280.4.G710
48. Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol (2011) 4(4):409–19. doi:10.1038/mi.2010.83
49. Trubiani O, Horenstein AL, Caciagli F, Caputi S, Malavasi F, Ballerini P. Expression of P2X7 ATP receptor mediating the IL8 and CCL20 release in human periodontal ligament stem cells. J Cell Biochem (2014) 115(6):1138–46. doi:10.1002/jcb.24756
50. Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood (2007) 109(4):1574–83. doi:10.1182/blood-2006-06-032961
51. Yang JS, Kim HJ, Kang SS, Kim KW, Kim DW, Yun CH, et al. TLR2, but not TLR4, plays a predominant role in the immune responses to cholera vaccines. J Leukoc Biol (2015) 98(4):661–9. doi:10.1189/jlb.4A1014-498R
52. Yang JS, Jeon JH, Jang MS, Kang SS, Ahn KB, Song M, et al. Vibrio cholerae OmpU induces IL-8 expression in human intestinal epithelial cells. Mol Immunol (2017) 93:47–54. doi:10.1016/j.molimm.2017.11.005
53. Fusunyan RD, Quinn JJ, Fujimoto M, MacDermott RP, Sanderson IR. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med (1999) 5(9):631–40.
54. Wang A, Si H, Liu D, Jiang H. Butyrate activates the cAMP-protein kinase A-cAMP response element-binding protein signaling pathway in Caco-2 cells. J Nutr (2012) 142(1):1–6. doi:10.3945/jn.111.148155
55. Ren H, Teng Y, Tan B, Zhang X, Jiang W, Liu M, et al. Toll-like receptor-triggered calcium mobilization protects mice against bacterial infection through extracellular ATP release. Infect Immun (2014) 82(12):5076–85. doi:10.1128/IAI.02546-14
56. Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature (1994) 371(6497):516–9. doi:10.1038/371516a0
57. Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, et al. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol (2007) 127(3):660–7. doi:10.1038/sj.jid.5700591
58. Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity (2013) 38(6):1142–53. doi:10.1016/j.immuni.2013.05.016
59. Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol (2008) 84(4):1159–71. doi:10.1189/jlb.0907612
60. Thakur BK, Dasgupta N, Ta A, Das S. Physiological TLR5 expression in the intestine is regulated by differential DNA binding of Sp1/Sp3 through simultaneous Sp1 dephosphorylation and Sp3 phosphorylation by two different PKC isoforms. Nucleic Acids Res (2016) 44(12):5658–72. doi:10.1093/nar/gkw189
61. Nepelska M, Cultrone A, Beguet-Crespel F, Le Roux K, Dore J, Arulampalam V, et al. Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLoS One (2012) 7(12):e52869. doi:10.1371/journal.pone.0052869
62. Brownell J, Bruckner J, Wagoner J, Thomas E, Loo YM, Gale M Jr, et al. Direct, interferon-independent activation of the CXCL10 promoter by NF-kappaB and interferon regulatory factor 3 during hepatitis C virus infection. J Virol (2014) 88(3):1582–90. doi:10.1128/JVI.02007-13
63. Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol (2015) 6:554. doi:10.3389/fimmu.2015.00554
64. Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU, Cuburu N, et al. Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. J Immunol (2011) 187(6):3044–52. doi:10.4049/jimmunol.1100402
65. Flach CF, Mozer M, Sundquist M, Holmgren J, Raghavan S. Mucosal vaccination increases local chemokine production attracting immune cells to the stomach mucosa of Helicobacter pylori infected mice. Vaccine (2012) 30(9):1636–43. doi:10.1016/j.vaccine.2011.12.111
66. Guo JH, Fan MW, Sun JH, Jia R. Fusion of antigen to chemokine CCL20 or CXCL13 strategy to enhance DNA vaccine potency. Int Immunopharmacol (2009) 9(7–8):925–30. doi:10.1016/j.intimp.2009.03.019
67. Shih NY, Yang HY, Cheng HT, Hung YM, Yao YC, Zhu YH, et al. Conditioning vaccination site with irradiated MIP-3alpha-transfected tumor cells enhances efficacy of dendritic cell-based cancer vaccine. J Immunother (2009) 32(4):363–9. doi:10.1097/CJI.0b013e31819d29d8
68. Kutzler MA, Wise MC, Hutnick NA, Moldoveanu Z, Hunter M, Reuter MA, et al. Chemokine-adjuvanted electroporated DNA vaccine induces substantial protection from simian immunodeficiency virus vaginal challenge. Mucosal Immunol (2016) 9(1):13–23. doi:10.1038/mi.2015.31
69. Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, et al. Stool microbiota and vaccine responses of infants. Pediatrics (2014) 134(2):e362–72. doi:10.1542/peds.2013-3937
70. Woo PC, Tsoi HW, Wong LP, Leung HC, Yuen KY. Antibiotics modulate vaccine-induced humoral immune response. Clin Diagn Lab Immunol (1999) 6(6):832–7.
71. Paineau D, Carcano D, Leyer G, Darquy S, Alyanakian MA, Simoneau G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol (2008) 53(1):107–13. doi:10.1111/j.1574-695X.2008.00413.x
72. Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, et al. Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr (2008) 138(1):123–9.
73. Matsuda F, Chowdhury MI, Saha A, Asahara T, Nomoto K, Tarique AA, et al. Evaluation of a probiotics, Bifidobacterium breve BBG-01, for enhancement of immunogenicity of an oral inactivated cholera vaccine and safety: a randomized, double-blind, placebo-controlled trial in Bangladeshi children under 5 years of age. Vaccine (2011) 29(10):1855–8. doi:10.1016/j.vaccine.2010.12.133
Keywords: cholera vaccine, short-chain fatty acids, butyrate, chemokines, intestinal epithelial cells
Citation: Sim J-R, Kang S-S, Lee D, Yun C-H and Han SH (2018) Killed Whole-Cell Oral Cholera Vaccine Induces CCL20 Secretion by Human Intestinal Epithelial Cells in the Presence of the Short-Chain Fatty Acid, Butyrate. Front. Immunol. 9:55. doi: 10.3389/fimmu.2018.00055
Received: 18 September 2017; Accepted: 09 January 2018;
Published: 29 January 2018
Edited by:
Eric Cox, Ghent University, BelgiumReviewed by:
Diane Bimczok, Montana State University, United StatesCopyright: © 2018 Sim, Kang, Lee, Yun and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Hyun Han, c2hoYW4tbWlAc251LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.