Stress-Activated Protein Kinases in Human Fungal Pathogens
- Faculty of Medicine, Institute for Cell and Molecular Biosciences, Newcastle University, Newcastle Upon Tyne, United Kingdom
The ability of fungal pathogens to survive hostile environments within the host depends on rapid and robust stress responses. Stress-activated protein kinase (SAPK) pathways are conserved MAPK signaling modules that promote stress adaptation in all eukaryotic cells, including pathogenic fungi. Activation of the SAPK occurs via the dual phosphorylation of conserved threonine and tyrosine residues within a TGY motif located in the catalytic domain. This induces the activation and nuclear accumulation of the kinase and the phosphorylation of diverse substrates, thus eliciting appropriate cellular responses. The Hog1 SAPK has been extensively characterized in the model yeast Saccharomyces cerevisiae. Here, we use this a platform from which to compare SAPK signaling mechanisms in three major fungal pathogens of humans, Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans. Despite the conservation of SAPK pathways within these pathogenic fungi, evidence is emerging that their role and regulation has significantly diverged. However, consistent with stress adaptation being a common virulence trait, SAPK pathways are important pathogenicity determinants in all these major human pathogens. Thus, the development of drugs which target fungal SAPKs has the exciting potential to generate broad-acting antifungal treatments.
Introduction
Stress responses are essential for pathogenic fungi to survive hostile environments encountered in the host (Brown et al., 2017), and it is apparent that stress-activated protein kinase (SAPK) pathways are central in mediating such responses and virulence in many fungal pathogens. SAPKs were first identified in the model yeast Saccharomyces cerevisiae where the High Osmolarity Glycerol (HOG) pathway was shown to regulate the cellular response to osmotic stress (Brewster et al., 1993). Subsequently, mammalian homologs, of the yeast Hog1 SAPK, namely p38 and JNK (c-Jun N-terminal kinase), were discovered (Galcheva-Gargova et al., 1994; Han et al., 1994). The high level of conservation between mammalian and yeast SAPKs is demonstrated by the fact that human p38 can complement the stress-sensitive phenotypes of the S. cerevisiae hog1Δ mutant (Han et al., 1994). It is now recognized that SAPKs are found in all eukaryotic cells, and are among the most evolutionarily conserved stress-signaling proteins (Nikolaou et al., 2009). There are three tiers of protein kinases within each SAPK module, comprising of a MAPKKK which phosphorylates and activates a MAPKK, which in turn phosphorylates and activates the terminal SAPK. Once active, the SAPK phosphorylates a range of different nuclear and cytoplasmic substrates thus triggering a myriad of distinct cellular responses.
The HOG pathway in S. cerevisiae is arguably the best characterized SAPK pathway, and using this as a reference we describe related SAPK pathways in three major fungal pathogens of humans; Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans.
Saccharomyces Cerevisiae Hog1p
Here, the role and regulation of Hog1p in response to osmotic stress will be summarized as, mechanistically, this is the best characterized system. However, it should be noted that S. cerevisiae Hog1p is also implicated in other stress responses including oxidative stress (Bilsland et al., 2004), cold stress (Hayashi and Maeda, 2006), hypoxia (Hickman et al., 2011), the toxic metabolite methylglyoxyl (Aguilera et al., 2005), arsenite (Lee and Levin, 2018), weak acid stress (Mollapour and Piper, 2007), and heat stress (Winkler et al., 2002).
The signaling pathways that regulate the relay of osmotic stress signals to Hog1p in S. cerevisiae is summarized in Figure 1. Within the three-tiered SAPK module, Hog1p is regulated by three MAPKKKs, the Ssk2p/22p orthologs and Stellp, and a single MAPKK Pbs2p (Posas and Saito, 1997). These upstream kinases regulate phosphorylation of Hog1p on threonine-174 and tyrosine-176 within the conserved TGY motif, which activates and induces the nuclear accumulation of the kinase (Ferrigno et al., 1998). Osmotic stress-sensing and signaling to the Hog1p SAPK module has been extensively characterized and involves two pathways that function redundantly (for excellent reviews see Saito and Posas, 2012; Brewster and Gustin, 2014). These pathways comprise the Sln1p two-component related signaling pathway, and the Sho1p pathway, which converge at the level of the Pbs2p MAPKK. Relevant for this review, it appears that whilst two-component related pathways are widely used to modulate SAPK activation in pathogenic fungi, Sho1-signaling to SAPKs is not universally conserved. Two-component phosphorelay pathways are widely used in bacteria to respond to environmental signals (Egger et al., 1997), but fungi have adapted a more complex three tier phosphorelay system. In S. cerevisiae, this comprises of the Sln1p histidine kinase, the Ypd1p phosphorelay protein, and the Ssk1p response regulator. Osmotic stress causes a loss of turgor pressure in the membrane, which inactivates the Sln1p histidine kinase and thus phosphorylation of Ssk1p via Ypd1p is inhibited (Posas et al., 1996). Dephosphorylated Ssk1p is a potent activator of the Ssk2p/22p MAPKKKs (Posas and Saito, 1998). Notably, loss of Sln1p or Ypd1p results in a lethal phenotype in S. cerevisiae due to accumulation of unphosphorylated Ssk1p and the consequent hyperactivation of Hog1p (Maeda et al., 1994). In the Sho1p branch, the Stellp MAPKKK phosphorylates Pbs2p (Tatebayashi et al., 2006), and Pbs2p acts as a scaffold to allow interaction between itself, Sho1p, Ste11p, and Hog1p (Posas and Saito, 1997). Many proteins have been implicated in the transmission of the osmosignal from Sho1p to Ste11p-Pbs2p (Tatebayashi et al., 2006), and there has been some debate regarding the osmosensor of the Sho1p pathway with two membrane-located mucins, Msb2p and Hkr1p, suggested as potential candidates (Tanaka et al., 2014). However, it has recently been shown that Sho1p is the osmosensor, with osmotic stress-induced structural changes in the transmembrane domains of Sho1p triggering binding to the cytoplasmic adaptor Ste50p, resulting in Hog1p activation (Tatebayashi et al., 2015). In addition to the two upstream branches promoting Hog1p phosphorylation, this kinase is also subjected to negative regulation via phosphatase action with the major phosphatases comprising of the phosphotyrosine phosphatases Ptp2p and Ptp3p (Wurgler-Murphy et al., 1997) and the 2C Ser/Thr phosphatase Ptc1p (Warmka et al., 2001).
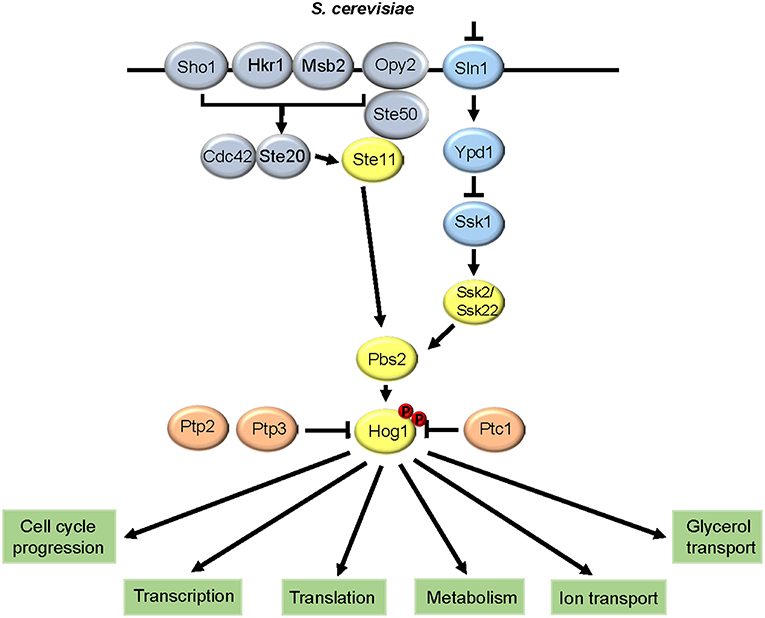
Figure 1. HOG pathway architecture in Saccharomyces cerevisiae. The two signaling branches that converge to regulate Hog1p, and the major downstream responses, are shown.
Following activation, Hog1p mounts a multi-layered response to osmotic stress via regulating both nuclear and cytoplasmic targets (Saito and Posas, 2012; Brewster and Gustin, 2014). Nuclear functions of Hog1p include the regulation of stress-protective gene expression, including genes involved in glycerol production and ion transport, via the Msn2p/4p, Smp1p and Hot1p transcriptional activators, and the Sko1p repressor (de Nadal and Posas, 2010). Of these perhaps the best characterized is the Sko1p ATF/CREB repressor which is a substrate of Hog1p and is phosphorylated following osmotic stress. This converts the Sko1p-Cyc8p-Tup1p complex from a repressor to an activator resulting in the recruitment of the SWI/SNF and SAGA chromatin-modifying complexes which induce binding of RNA polymerase II and transcription of osmotic stress genes (Proft and Struhl, 2002). In addition, Hog1p regulates delays in the cell cycle at G1, S, and G2 phases to permit stress adaptation prior to cell-cycle progression (Clotet and Posas, 2007). Cytoplasmic functions of Hog1p include the regulation of metabolic enzymes (Dihazi et al., 2004), and the closing of the Fps1p aquaglyceroporin (Lee et al., 2013). Interestingly, although nuclear accumulation of Hog1p is required for transcription of osmotic stress genes, preventing nuclear accumulation does not impact osmotic stress resistance (Westfall et al., 2008). This suggests that cytoplasmic functions of Hog1p, rather than transcriptional responses in the nucleus, are more important in promoting osmotic stress resistance in S. cerevisiae.
Candida albicans Hog1
A number of Candida spp. are pathogens of humans with C. albicans being the most important causing 400,000 life threatening systemic infections per annum (Brown et al., 2012). The role and regulation of the Hog1 pathway in C. albicans is summarized in Figure 2. HOG1 was first identified in C. albicans in 1996 as a gene that could rescue the osmotic stress sensitive phenotype of the S. cerevisiae hog1Δ mutant (San Jose et al., 1996). Subsequently, C. albicans Hog1 has been found to be activated and/or promote resistance to diverse stress conditions likely to be encountered in the host or during antimicrobial therapy. For example, Hog1 is important for cellular responses to osmotic and oxidative stresses (Alonso-Monge et al., 2003; Smith et al., 2004), nitrosative stress (Herrero-de-Dios et al., 2018), metal availability (Kaba et al., 2013), various drugs (Smith et al., 2004; Kelly et al., 2009), antimicrobial peptides (Vylkova et al., 2007; Argimon et al., 2011; Hayes et al., 2013), increased glucose levels (Rodaki et al., 2009), and photodynamic inactivation (Chien et al., 2018). Whereas hog1Δ cells generally display impaired resistance to the aforementioned stresses, loss of Hog1 increases resistance to cell wall damaging agents such as calcofluor white and congo red (Alonso-Monge et al., 1999). This is due to inappropriate activation of the Cek1 MAPK pathway in hog1Δ cells via the cross talk phenomenon (Eisman et al., 2006).
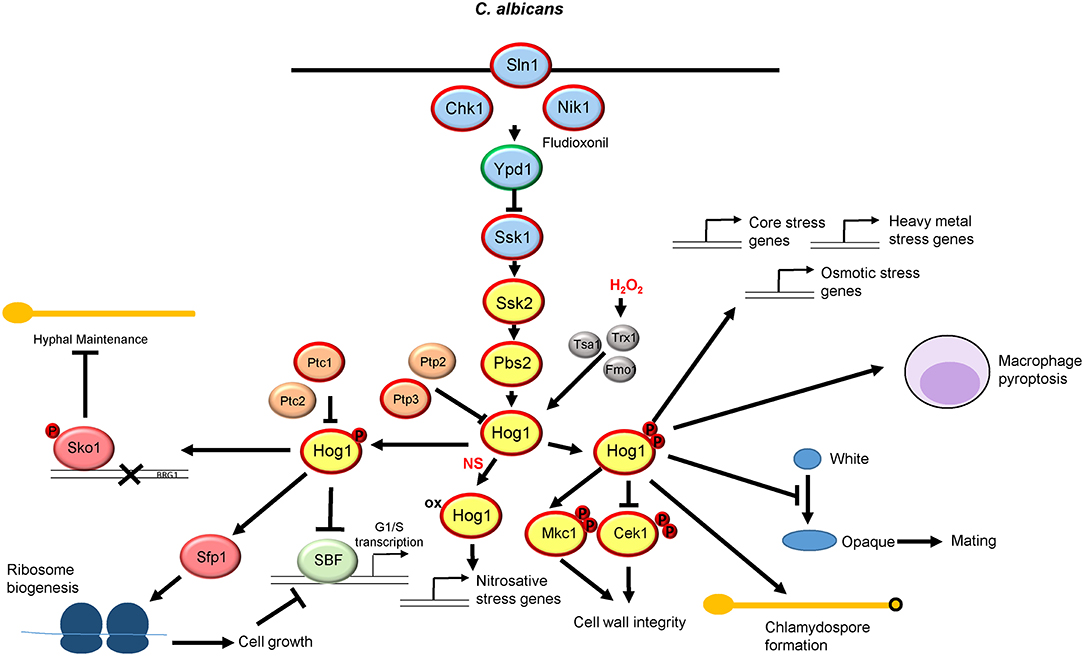
Figure 2. The Hog1 SAPK pathway in Candida albicans. Both the signaling proteins that regulate Hog1 and the downstream responses are shown. See text for details. Those proteins circled in red are required for C. albicans virulence, whereas Ypd1 circled in green promotes virulence upon inactivation.
In addition to stress resistance, Hog1 is also implicated in a multitude of cellular processes in C. albicans including chlamydospore production (Alonso-Monge et al., 2003) and morphogenetic switching (Alonso-Monge et al., 1999; Enjalbert et al., 2006; Su et al., 2013), with hog1Δ cells displaying filamentous growth in the absence of any morphogenetic cues (Enjalbert et al., 2006). Hog1 also impacts on respiratory metabolism (Alonso-Monge et al., 2009), cell cycle regulation (Correia et al., 2016; Sellam et al., 2019), and white-opaque switching and mating (Liang et al., 2014). Hog1 function is also intimately connected in regulating processes triggered following Candida-phagocyte interactions including induction of macrophage pyroptosis (O'Meara et al., 2018), and the dynamic reorganization of the fungal cell wall initiated in response to neutrophil extracellular trap (NET)-mediated damage (Hopke et al., 2016), the latter of which may be linked to the deregulation of the Cek1 MAPK in hog1Δ cells (Eisman et al., 2006).
As perhaps expected due to the involvement of Hog1 in multiple processes in C. albicans, this SAPK is essential for virulence. This was first shown in 1999 when, in a murine systemic model of candidiasis, mice injected with hog1Δ cells were found to survive significantly better than those injected with wild type cells (Alonso-Monge et al., 1999). Subsequently, Hog1 has also been found to be important for fungal survival following phagocytosis (Arana et al., 2007), and in promoting colonization of the mouse gastrointestinal tract (Prieto et al., 2014). As Hog1 regulates the yeast to hyphal switch, which is important for C. albicans pathogenesis (Lo et al., 1997), it is possible that this underlies Hog1-mediated virulence. However, a Hog1-pathway mutant that showed impaired stress resistance but no defect in morphological switching displayed reduced virulence analogous to hog1Δ cells suggesting that Hog1 contributes to virulence independently of its role in morphological switching (Cheetham et al., 2011).
Within the C. albicans SAPK module, Hog1 is activated via the Pbs2 MAPKK and the Ssk2 MAPKKK. Thus, in response to many of the stresses outlined above, Hog1 becomes phosphorylated on the conserved TGY motif in a Pbs2 and Ssk2-dependent manner. Consequently C. albicans cells lacking PBS2 or SSK2 largely phenocopy hog1Δ cells (Arana et al., 2005; Cheetham et al., 2007, 2011). Interestingly, homology between C. albicans Pbs2 and S. cerevisiae Pbs2p appears to be restricted to the C-terminus, with the N-terminal region of C. albicans Pbs2 being considerably shorter than that of the S. cerevisiae protein (Cheetham et al., 2007). However, the activating phosphorylation sites are conserved and mutation to non-phosphorylatable alanine residues generates identical phenotypes to those displayed by pbs2Δ cells (Cheetham et al., 2011). In contrast to S. cerevisiae, Pbs2 is activated by a sole MAPKKK Ssk2 in C. albicans (Cheetham et al., 2007). Although homologs of the Sho1 osmosensing branch are conserved in this fungal pathogen, neither Sho1 nor the downstream Ste11 MAPKKK function to relay osmotic stress signals to Hog1 (Roman et al., 2005; Cheetham et al., 2007). It was thought that this may be due to the truncated N-terminal region of C. albicans Pbs2 (Cheetham et al., 2007) which lacks the Sho1-binding domain present in S. cerevisiae Pbs2p (Tatebayashi et al., 2003). However, a chimeric protein comprising the N-terminus of S. cerevisiae Pbs2p fused to the C-terminus of C. albicans Pbs2, whilst functional, was unable to activate Hog1 in response to osmotic stress in the absence of Ssk2 (Cheetham et al., 2011). Collectively, these findings illustrate that the Sho1-Ste11 pathway in C. albicans does not respond to changes in osmolarity.
As in S. cerevisiae, the C. albicans Hog1 module is regulated by a two-component related phosphorelay system (Smith et al., 2010). However, in contrast to S. cerevisiae, C. albicans has three sensor histidine kinases, Sln1, Nik1, and Chk1 although their mechanism of action is unclear. C. albicans Sln1 is homologous to S. cerevisiae Sln1p and contains two transmembrane domains indicating a conserved plasma membrane location (Salas-Delgado et al., 2017). C. albicans Sln1 is able to rescue the lethality associated with loss of SLN1 in S. cerevisiae although SLN1 is not an essential gene in C. albicans (Nagahashi et al., 1998). In addition, loss of SLN1 causes constitutive activation of Hog1 in C. albicans as in S. cerevisiae (Roman et al., 2005). Collectively these observations indicate that C. albicans Sln1 likely functions as an osmosensor, however 2-component independent mechanisms also exist in the relay of osmotic stress signals to Hog1 (Chauhan et al., 2003). The C. albicans Nik1 histidine kinase is predicted to be cytoplasmic and, similar to other Nik1-related kinases, contains a number of HAMP (Histidine kinases, Adenylate cyclases, Methyl-accepting proteins, and Phosphatases) domains. Notably, efforts to generate a nik1Δ/sln1Δ double mutant proved unsuccessful (Yamada-Okabe et al., 1999), possibly implying functional redundancy between these kinases.
The function of Nik1 in Hog1 regulation is unclear. A recent high-throughput screen for pharmacologically active compounds that enhance the fungicidal activity of the cell wall targeting echinocandin, caspofungin, found that the metal chelator DTPA demonstrated the greatest synergistic activity by chelating magnesium (Polvi et al., 2016). Suppressor mutants were isolated that displayed resistance to the caspofungin and DTPA drug combination and remarkably, three of the four sequenced mutants were found to contain mutations in the NIK1 gene. Such NIK1 mutations impaired Hog1 activation in response to caspofungin and DTPA, which likely underlies the stress-resistant phenotype, as cells lacking Hog1 were also found to be resistant to this drug combination (Polvi et al., 2016). In a similar vein, the phenylpyrrole fungicide fludioxonil, used on crops worldwide, appears to exert its antifungal effects via activation of the Hog1 signaling pathway in a mechanism involving the Nik1 class of histidine kinases. For example, expression of C. albicans NIK1 in S. cerevisiae renders this yeast hypersensitive to fludioxonil in a Hog1-dependent manner (Buschart et al., 2012). Furthermore, the HAMP domains within C. albicans Nik1 are important for the lethal activation of Hog1p in S. cerevisiae following fludioxonil treatment (Buschart et al., 2012). Thus, Nik1 appears to drive activation of Hog1 in response to distinct antifungals which promotes fungicidal activity.
The third histidine kinase in C. albicans, Chk1, is also predicted to be cytoplasmic and is a homolog of Mak2 and Mak3 in Schizosaccharomyces pombe. In S. pombe, Mak2 and Mak3 relay oxidative stress signals to the Sty1 SAPK module (Buck et al., 2001), but Chk1 is dispensable for hydrogen peroxide-induced activation of Hog1 in C. albicans (Li et al., 2004; Roman et al., 2005). However, chk1Δ cells show sensitivity to oxidative stress (Li et al., 2004), and using a LacZ reporter, it was found that transcription of CHK1 was increased in response to oxidative stress (Li et al., 2004). Interestingly, Chk1 also appears to modulate the C. albicans cell wall (Li et al., 2004), but it is unknown if this is mediated via Hog1 regulation. Importantly, all three histidine kinases in C. albicans are needed for virulence (Calera et al., 1999; Yamada-Okabe et al., 1999; Selitrennikoff et al., 2001; Torosantucci et al., 2002), which due to their absence in metazoans makes such proteins potential antifungal candidates (Shor and Chauhan, 2015).
As in S. cerevisiae, C. albicans has a single phosphorelay protein which is homologous to S. cerevisiae Ypd1p. Although deletion of YPD1 is lethal in S. cerevisiae (Maeda et al., 1994), due to the constitutive activation of Hog1 leading to apoptosis (Vendrell et al., 2011), this is not the case in C. albicans. Deletion of YPD1 does trigger the constitutive activation of C. albicans Hog1 (Mavrianos et al., 2014; Day et al., 2017b), but cells adapt to this in the short term by inducing protein tyrosine phosphatase genes that prevent lethal levels of Hog1 phosphorylation (Day et al., 2017b). In addition, following long term sustained Hog1 phosphorylation, C. albicans mounts a distinct mechanism that reduces Hog1 phosphorylation such that ypd1Δ cells become phenotypically indistinguishable from wild-type cells (Day et al., 2017b). Interestingly, and perhaps surprisingly, down-regulation of YPD1 expression in a murine model of systemic candidiasis actually enhanced the virulence of C. albicans. Whilst the mechanism underlying the enhanced virulence triggered by YPD1 loss requires investigation, in contrast to previous suggestions (Shor and Chauhan, 2015), this indicates that Ypd1 is not a suitable antifungal drug target in C. albicans (Day et al., 2017b).
Downstream of Ypd1 is the Ssk1 response regulator. In S. cerevisiae, unphosphorylated, but not phosphorylated, Ssk1p drives the activation of the Ssk2p/Ssk22p MAPKKKs (Posas and Saito, 1998). Interestingly, in C. albicans, deletion of the analogous SSK1 gene does not impact on Hog1 activation in response to osmotic stress. Instead, Ssk1 was shown to impact on Hog1 activation following oxidative stress, and consistent with this ssk1Δ cells display sensitivity to oxidative stress (Chauhan et al., 2003). However, it appears that none of the histidine kinases in C. albicans sense oxidative stress to regulate phosphorelay to Ssk1, as none impact on oxidative-stress induced activation of Hog1 (Roman et al., 2005). Thus, Ssk1 may relay oxidative stress signals to Hog1 in a mechanism independent of two-component signaling. Importantly, Ssk1 is essential for C. albicans virulence (Calera et al., 2000), and thus is potentially a more suitable antifungal target than Ypd1.
Two component independent mechanisms are also clearly involved in Hog1 activation in C. albicans. The redox-sensitive thioredoxin peroxidase, Tsa1, and the thioredoxin, Trx1, which reduces oxidized Tsa1, are required for H2O2-induced Hog1 activation (da Silva Dantas et al., 2010). However, Trx1 likely regulates Hog1 in a distinct mechanism than Tsa1, as the cysteine residues of Tsa1 regulated by Trx1 are not required for Hog1 activation (da Silva Dantas et al., 2010). In S. pombe, the Tsa1 ortholog, Tpx1, is similarly required for activation of the Sty1 SAPK in response to oxidative stress via a mechanism involving the formation of an intermolecular disulphide bond with Sty1 (Veal et al., 2004). However, C. albicans Hog1 is only oxidized following nitrosative stress and not oxidative stress (Herrero-de-Dios et al., 2018), suggesting that Tsa1 does not form a mixed disulphide with Hog1 following H2O2 stress. In addition to Tsa1/Trx1 in regulating H2O2-induced Hog1 activation, deletion of a mitochondrial biogenesis factor, Fzo1, also impairs oxidative stress-induced Hog1 phosphorylation (Thomas et al., 2013). Thus, mitochondrial function may also impact on oxidative stress induced activation of Hog1. C. albicans Hog1 is also negatively regulated by the Ptp2 and Ptp3, and Ptc1, phosphatases (Su et al., 2013; Sellam et al., 2019). Finally, although the pathways described above regulate Hog1 phosphorylation levels, a recent study revealed that oxidation of Hog1 is implicated in nitrosative-stress signaling (Herrero-de-Dios et al., 2018). However, the mechanism by which oxidation modulates Hog1 activity requires further investigation.
Regarding downstream responses regulated by Hog1, transcript profiling analysis revealed Hog1 to regulate the C. albicans transcriptome both in the absence and presence of stress (Enjalbert et al., 2006). Under basal conditions Hog1 represses subsets of stress-related genes and hyphae specific genes. The upregulation of hyphae specific genes is consistent with the filamentous phenotype of this mutant (Enjalbert et al., 2006), whereas the upregulation of genes involved in reactive oxygen species (ROS) metabolism may reflect the observation that hog1Δ cells have higher levels of intracellular ROS than wild-type cells (Alonso-Monge et al., 2009). Hog1 also plays a major role in the induction of core stress genes, which are commonly upregulated in response to osmotic, oxidative, and cadmium-imposed heavy metal stress, in addition to genes specifically induced in response to osmotic and heavy metal stress (Enjalbert et al., 2006). Intriguingly, a recent study revealed that in contrast to that reported in S. cerevisiae (Westfall et al., 2008), stress-induced gene expression in C. albicans can occur when the nuclear accumulation of Hog1 is prevented (Day et al., 2017a). Notably preventing Hog1 nuclear accumulation also had no impact on C. albicans virulence, suggesting that cytoplasmic functions of Hog1 may be more important (Day et al., 2017a). Furthermore, although Hog1 also plays a major role in the regulation of nitrosative stress genes, Hog1 is not noticeably phosphorylated following nitrosative stress (Herrero-de-Dios et al., 2018). Instead, Hog1 is oxidized following nitrosative stress suggesting that this post-translational modification can also modulate Hog1 function in C. albicans (Herrero-de-Dios et al., 2018). However, in contrast to the aforementioned stresses, Hog1 is largely dispensable for oxidative stress-induced gene expression (Enjalbert et al., 2006), even though hog1Δ cells are sensitive to ROS (Alonso-Monge et al., 2003). This suggests that Hog1 plays a non-transcriptional role in promoting oxidative stress tolerance in C. albicans. In this regard, it is noteworthy that Hog1 promotes the recovery from oxidative stress-induced cell cycle arrest (Correia et al., 2016). Furthermore, following oxidative stress the cell wall integrity MAPK Mkc1 is rapidly phosphorylated in a Hog1-dependent mechanism. However, this is unlikely to underlie the role of Hog1 in H2O2 resistance, as Mkc1 is dispensable for oxidative stress tolerance (Navarro-Garcia et al., 2005).
Regarding downstream targets of Hog1, two genes that encode putative substrates for the SAPK are transcriptionally induced following osmotic stress in a Hog1-dependent manner. The first is the Rck2 serine-threonine protein kinase, homologs of which are phosphorylated by the Hog1p and Sty1 SAPKs in the model yeasts S. cerevisiae and S. pombe, respectively (Bilsland-Marchesan et al., 2000; Smith et al., 2002). Interestingly deletion of RCK2 in C. albicans renders cells sensitive to rapamycin, a phenotype shared by hog1Δ cells (Li et al., 2008). The second is Sko1, a CRE-binding transcriptional repressor that is a well-characterized Hog1p-target in S. cerevisiae (Proft and Struhl, 2002). Sko1 is phosphorylated by Hog1 (Rauceo et al., 2008) and this transcription factor regulates a subset of Hog1-dependent osmotic stress genes (Marotta et al., 2013). Moreover, Hog1 mediated regulation of Sko1 is linked to the filamentous phenotype exhibited by hog1Δ cells. Basal levels of Hog1 phosphorylation are needed to regulate Sko1 to dampen the expression of BRG1, a transcription factor required for the induction of hyphal genes (Su et al., 2013). In cells lacking Hog1, unphosphorylated Sko1 no longer binds to the BRG1 promoter, which allows for BRG1 expression and filamentation (Su et al., 2013). However, other transcriptional targets of Hog1p in S. cerevisiae, such as Msn2p and Msn4p, appear to be functionally reassigned in C. albicans (Nicholls et al., 2004). In a recent study, roles for Hog1 linking cell growth and division in C. albicans via transcription factor regulation have been uncovered (Sellam et al., 2019). Cells lacking Hog1 are significantly smaller than wild-type cells via a mechanism in which basal levels of Hog1 activation inhibit the SBF G1/S transcription factor complex thus delaying transition into S phase of the cell cycle. The Ptc1 and Ptc2 phosphatases, by modulating Hog1 phosphorylation, also govern the timing of Start. In parallel, Hog1 physically interacts with Sfp1, the transcription factor that regulates ribosome biogenesis, and recruits Sfp1 to ribosome biogenesis genes (Sellam et al., 2019). This interaction is abolished by stress providing a mechanism by which the timing of Start is regulated in part by modulation of the Hog1-Sfp1 interaction.
Aspergillus Fumigatus SakA and MpkC
The genus aspergillus consists of several 100 species with Aspergillus fumigatus being the most important fungal pathogen of humans, causing ~65% of all invasive fungal infections in humans (Brown et al., 2012). Intriguingly, A. fumigatus contains two homologs of Hog1 known as SakA and MpkC, which share 68.4% identity. The role and regulation of the SakA and MpkC pathways is summarized in Figure 3. Analysis of single mutants in SakA revealed roles in adaptation to all main antifungal classes (polyenes, azoles, and echinocandins) and cold stress tolerance (Kim et al., 2012; Wong Sak Hoi et al., 2012; Altwasser et al., 2015), whereas MpkC function was linked to carbon source utilization (Reyes et al., 2006). Subsequently, a comparison of single and double mutants revealed that SakA and MpkC coordinate osmotic stress resistance with both displaying osmotic stress-induced nuclear accumulation albeit with different kinetics (Bruder Nascimento et al., 2016). However, in response to other stresses, SakA seems to be the most important, as ΔsakA and ΔmpkC ΔsakA mutants, but not the ΔmpkC strain, display notable increased sensitivity to ROS, cell wall damaging agents, and the cell wall-targeting antifungals caspofungin and nikkomycin Z (Bruder Nascimento et al., 2016). Furthermore, ΔsakA and ΔmpkC ΔsakA strains displayed alterations in cell wall composition and were less adherent than the wild-type strain (Bruder Nascimento et al., 2016). Such phenotypes may be linked to the interesting observation that activation of the MpkA cell wall integrity MAPK pathway in response to osmotic or cell wall stress was largely dependent on SakA and MpkC (Bruder Nascimento et al., 2016). Most importantly, SakA and MpkC likely play redundant roles in the virulence of this important fungal pathogen, as only the ΔmpkC ΔsakA strain and not the single mutants displayed highly attenuated virulence in a mouse neutropenic model of invasive pulmonary aspergillosis (Bruder Nascimento et al., 2016). Functioning upstream of Hog1 is a single MAPKK PbsB and, as in C. albicans, a single MAPKKK SskB. SakA phosphorylation is abolished in ΔpbsB cells (Ji et al., 2012) and ΔsskB cells (de Castro et al., 2014), and both mutants display acute sensitivity to cationic stress similar to that seen for ΔsakA mutants (de Castro et al., 2014). Furthermore, similar to cells lacking both SakA and MpkC, the ΔsskB mutant was found to display significantly attenuated virulence in an invasive pulmonary model of aspergillosis (de Castro et al., 2014) emphasizing the importance of SAPK signaling for A. fumigatus survival in the host.
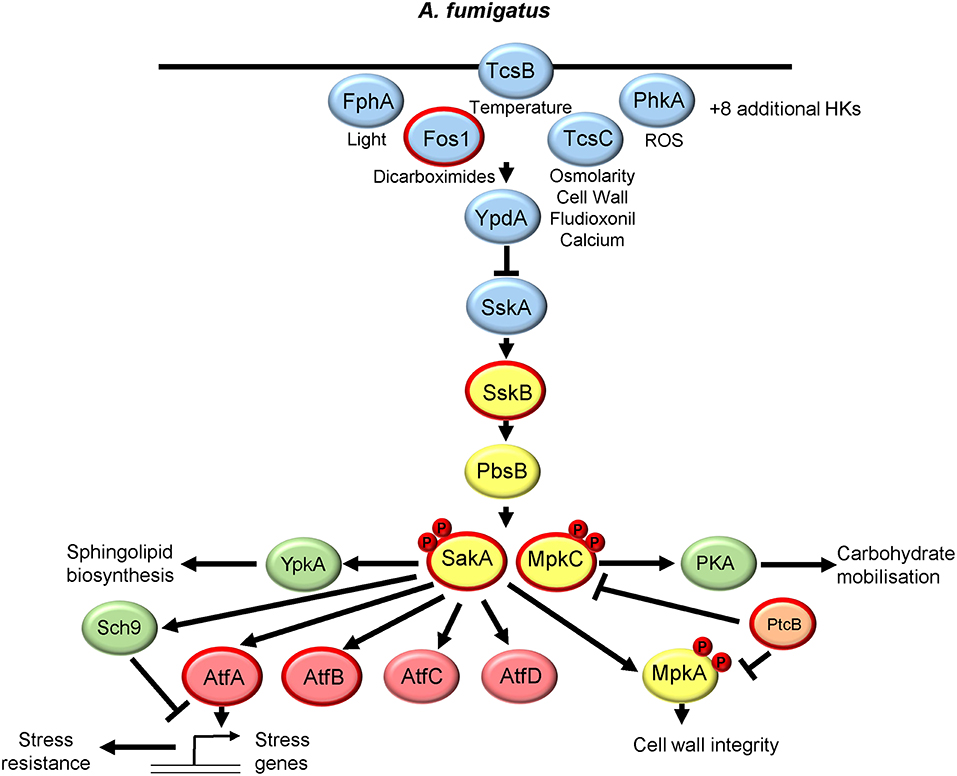
Figure 3. The SakA and MpkC SAPK pathway in Aspergillus fumigatus. Both the signaling proteins that regulate SakA and MpkC and the downstream responses are shown. See text for details. Those proteins circled in red are required for A. fumigatus virulence.
Similar to that first reported in S. cerevisiae, in A. fumigatus two-component related phosphorelay systems likely act upstream of SskB to regulate SakA. However, filamentous fungi have more histidine kinases compared to yeasts, with the A. fumigatus genome containing 13 histidine kinases (Chapeland-Leclerc et al., 2015). The first to be characterized was Fos1/TcsA, which contains a PAS domain, with the Δfos1 strain displaying high levels of resistance to dicarboximide fungicides (Pott et al., 2000) and highly attenuated virulence in a murine model of systemic aspergillosis (Clemons et al., 2002). A homolog of Sln1, named TcsB, is also present and this is the only histidine kinase predicted to be located at the cell wall (Du et al., 2006), and regulates SakA in response to temperature stress (Ji et al., 2012). The histidine kinase associated with most phenotypes in A. fumigatus is a homolog of C. albicans Nik1, TcsC, which has six HAMP domains. Phenotypes linked to TcsC include reduction of conidiation, increased tolerance to cell wall-perturbing reagents and the fungicide fludioxonil, and enhanced sensitivity to osmotic stress stimuli and calcium stress (McCormick et al., 2012; Hagiwara et al., 2013; de Castro et al., 2014). Conflicting reports exist as to whether TcsC regulates SakA phosphorylation in response to such stresses (McCormick et al., 2012; Hagiwara et al., 2013), although the basal level of SakA phosphorylation is increased in ΔtcsC cells indicating that the stress-induced inactivation of TcsC triggers SakA phosphorylation (Hagiwara et al., 2013). A. fumigatus also has a homolog of the red light receptor histidine kinase, FphA, which in A. nidulans causes light dependent phosphorylation of SakA (Yu et al., 2016). Interestingly, FphA also regulates photoresponsive behaviors of A. fumigatus (Fuller et al., 2013), which raises the possibility that some of these responses may be mediated through SakA. Recently, a systematic analysis of 11 A. fumigatus histidine kinase gene deletions has been undertaken (Chapeland-Leclerc et al., 2015). Although most of the previously uncharacterized histidine kinase mutants failed to display strong phenotypes, cells lacking the class X histidine kinase PhkA displayed a clear hypersensitivity to oxidative stress agents (Chapeland-Leclerc et al., 2015). Notably, PhkA is homologous to the oxidative stress sensing Mak2 and Mak3 histidine kinases in S. pombe (Buck et al., 2001), although it remains to be tested whether PhkA relays oxidative stress signals to SakA.
Downstream of the histidine kinases, a homolog of the Ypd1 phosphorelay protein is present in the A. fumigatus genome but remains uncharacterized. However, a homolog of the Ssk1 response regulator SskA has been characterized, with ΔsskA cells displaying sensitivity to osmotic stress and no detectable phosphorylation of the SakA SAPK (Hagiwara et al., 2013). This implies that activation of Sak1 in response to osmotic stress is entirely dependent on the two-component related signaling system, in contrast to that seen in C. albicans in which osmotic stress signaling to the Hog1 SAPK occurs independently of Ssk1 (Chauhan et al., 2003).
Regarding two-component independent regulation of SakA/MpkC activation, the phosphatase PtcB, a homolog of the Ptc2p-Ptc3p type 2C serine/threonine phosphatases that dephosphorylate S. cerevisiae Hog1p, regulates SakA (Winkelstroter et al., 2015). Cells lacking PtcB display high basal levels of SakA phosphorylation and higher basal and stress-induction of the osmostress genes catA, dprA, and dprB (Winkelstroter et al., 2015). In addition, the ΔptcB strain showed impaired virulence in a mouse model of invasive pulmonary aspergillosis (Winkelstroter et al., 2015), but whether this is due to SakA deregulation is unclear as PtcB also regulates the activity of the MpkA cell wall integrity MAPK pathway (Winkelstroter et al., 2015). Interestingly the calcium responsive CrzA transcription factor has been linked to SakA activation in A. fumigatus, as phkB histidine kinase and sskB MAPKKK gene expression require CrzA (de Castro et al., 2014). In addition, whilst basal levels of SakA phosphorylation are increased in ΔcrzA cells, no further osmotic stress increase in SAPK phosphorylation is seen (de Castro et al., 2014).
Turning to the downstream responses elicited following SakA/MpkC activation, transcript profiling of single and double SAPK mutants revealed independent and overlapping roles for SakA and MpkC, with SakA playing a predominant role (Pereira Silva et al., 2017). Genes that require SakA for osmotic stress induction showed enrichment in the GO terms; cellular response to oxidative and osmotic stresses, trehalose catabolic process, and two-component signal transduction. In contrast, MpkC regulated genes did not display any significant GO term enrichment (Pereira Silva et al., 2017). However both SAPKs regulated the osmotic stress-repression of genes involved in cell cycle, metabolism and ribosome biogenesis (Pereira Silva et al., 2017). Interestingly, MpkC and SakA influence the expression of genes within the two-component related phosphorelay, and the SAPK module, which regulate their activation (Pereira Silva et al., 2017). In addition, a large number of kinases and transcription factors, some of which have previously been linked to SAPK function in S. cerevisiae or S. pombe, were found to be induced in a SAPK-dependent manner. One such kinase, SchA, is an ortholog of Sch9p, which is a chromatin-associated transcriptional activator of osmostress-responsive genes in S. cerevisiae (Pascual-Ahuir and Proft, 2007). However, in A. fumigatus cells lacking SchA, SakA phosphorylation, and SakA-dependent gene expression are sustained following osmotic stress, and cells lacking both Sch9 and SakA are much more sensitive to osmotic stress than the single mutants (de Castro et al., 2014). This suggests that SchA has a different role in mediating SAPK responses than in S. cerevisiae. In addition, the regulatory (PkaR) and catalytic (PkaC) subunits of protein kinase A were found to be induced in response to osmotic stress in a SakA and MpkA dependent manner (Pereira Silva et al., 2017). Subsequently, PKA activity was shown to be significantly reduced in ΔsakA and ΔsakA ΔmpkC strains and, intriguingly, co-immunoprecipitation experiments revealed that PKA and SakA physically interact (de Assis et al., 2018). Specifically, under non-stress conditions both regulatory and catalytic PKA subunits interact with SakA, but following osmotic stress the PkaR subunit dissociates, which is suggested to trigger PKA activity and trehalose and glycogen degradation (de Assis et al., 2018). Thus, SakA/MpkC have both transcriptional and posttranscriptional roles in regulating PKA activity in A. fumigatus. Interestingly, SakA has also recently been reported to physically interact with the AGC kinase YpkA which regulates sphingolipid synthesis (Fabri et al., 2018). Regarding potential downstream transcription factors, orthologs of the S. pombe SAPK substrate Atf1 (Lawrence et al., 2007), namely AtfA, B, C, and D, were induced in wild-type cells in a SakA dependent manner following osmotic stress (Pereira Silva et al., 2017). AtfA is the most important for stress tolerance in both A. fumigatus conidia (Hagiwara et al., 2014), and during mycelial growth (Pereira Silva et al., 2017). Furthermore, AtfA, and to a lesser extent AtfB, contribute to virulence (Pereira Silva et al., 2017). Whether such transcription factors are substrates for the SakA/MpkC SAPKs, as in S. pombe, remains to be tested. Moreover, as there are many additional transcription factors and kinases that are regulated by SakA/MpkC, much is still to be learnt about the SAPK-mediated regulatory networks in this important fungal pathogen of humans.
Cryptococcus Neoformans Hog1
There are seven species of Cryptococcus, but research into Hog1 signaling has thus far been restricted to C. neoformans (Serotype A) and C. deneoformans (Serotype D) (Hagen et al., 2015). C. neoformans (Serotype A) is the major species responsible for cryptococcal meningitis, resulting in over 180,000 deaths each year (Rajasingham et al., 2017). The role and regulation of the C. neoformans serotype A Hog1 pathway is summarized in Figure 4. The core SAPK signaling module is conserved comprising of the Hog1 SAPK, the Pbs2 MAPKK and, as in C. albicans and A. fumigatus, a single MAPKKK Ssk2 (Bahn et al., 2007). Intriguingly, in the majority of C. neoformans serotype A strains tested and in many, but not all, C. deneoformans serotype D strains, Hog1 regulation is unique in that Hog1 is constitutively phosphorylated under non-stress conditions and is actively dephosphorylated in response to various stress stimuli (Bahn et al., 2005). In contrast, in the serotype D strains that do not show high basal levels of Hog1 phosphorylation, typical stress-induced increases in Hog1 phosphorylation are seen. Notably, strains displaying high basal levels of Hog1 phosphorylation are generally more stress resistant. An elegant genetic approach identified polymorphisms in the SSK2 gene as responsible for the variation in basal levels of Hog1 phosphorylation in different strain backgrounds, one of which (L240F) resides in the putative Ssk1 binding domain (Bahn et al., 2007). Interchange of SSK2 alleles between strains showing either high or low Hog1 phosphorylation levels verified that polymorphisms in Ssk2 underlie the differential regulation of the Hog1 MAPK pathway (Bahn et al., 2007).
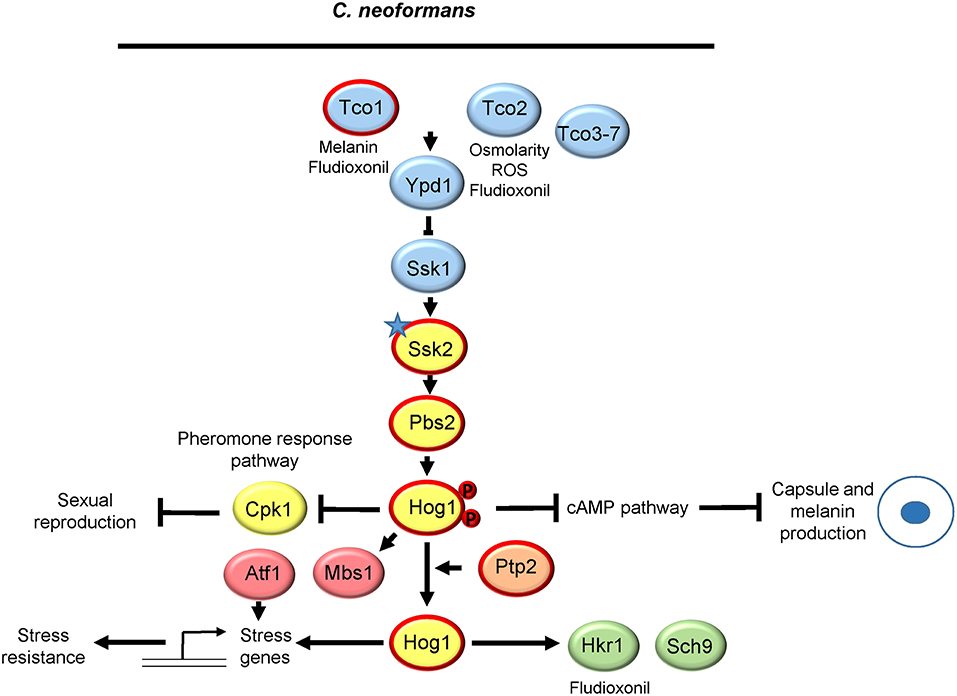
Figure 4. The Hog1 SAPK pathway in Cryptococcus neoformans. Both the signaling proteins that regulate Hog1 and the downstream responses are shown. The polymorphism in Ssk2 that drives Hog1 activation in the absence of stress is indicated with a star. See text for details. Those proteins circled in red are required for C. neoformans virulence.
Cryptococcus neoformans Hog1 regulates a panoply of virulence attributes. With regard to stress resistance the Hog1 pathway promotes resistance to stresses likely encountered in the host and the environment, including osmotic and oxidative stresses, heat stress, UV irradiation, and HU and MMS-imposed genotoxic stresses, antifungal drugs including amphotericin and the toxic metabolic by-product methylglyoxal (Bahn et al., 2005, 2006, 2007; Lee et al., 2014a). It is intriguing that many such stress stimuli trigger the dephosphorylation of Hog1, indicating that stress-induced inactivation of Hog1 promotes stress resistance in C. neoformans. A common response to stress to conserve energy is to inhibit the energy demanding process of ribosome biogenesis, and in C. neoformans Hog1 function is important for the rapid degradation of ribosomal protein transcripts triggered by glucose starvation (Banerjee et al., 2016). Here it is postulated that ROS generated following glucose starvation may be the Hog1 regulatory signal (Banerjee et al., 2016). In contrast to processes requiring Hog1, cells lacking components of the Hog1 module are much more resistant to the antifungal agent fludioxonil (Bahn et al., 2005, 2007) which is consistent with the mode of action of fludioxonil mentioned previously, in stimulating the lethal hyperactivation of fungal SAPK pathways (Kojima et al., 2004). Interestingly, the high basal level of Hog1 phosphorylation seen in C. neoformans negatively impacts the production of two key virulence traits, capsule and melanin, likely via cross talk with the cAMP pathway (Bahn et al., 2005). Similarly, Hog1 also inhibits sexual reproduction via cross talk with the pheromone responsive Cpk1 MAPK pathway (Bahn et al., 2005). Consequently, hog1Δ, pbs2Δ, and ssk2Δ mutants all display enlarged capsule, increased melanin content, and enhanced mating proficiency (Bahn et al., 2005, 2007). Interestingly, despite the hyperproduction of capsule and melanin, deletion of core components of the MAPK module impairs C. neoformans virulence (Bahn et al., 2005, 2007).
As in other fungi, a two-component related phosphorelay system functions upstream of the SAPK module to relay environmental signals to C. neoformans Hog1 (Bahn, 2008). There are seven histidine kinases in C. neoformans, Tco1-7, which potentially facilitate sensing of multiple environmental cues (Bahn et al., 2006). Common to eukaryotic histidine kinases most contain a single histidine kinase and receiver domain although, intriguingly, Tco2 has two of each domain (Bahn et al., 2006). However, there is no ortholog of the Sln1 osmosensing histidine kinase, and none of the seven present histidine kinases possess a transmembrane domain suggesting that they sense intracellular stress signals (Bahn et al., 2006). Phenotypic analysis of Tco mutants identified Tco1 and Tco2 to have overlapping and specific functions in Hog1 regulation. Tco1 shows structural similarity to CaNik1 whereas Tco2 is unique. Loss of Tco2 resulted in impaired resistance to osmotic and oxidative stress and toxic metabolites, and the osmotic stress-induced decrease in Hog1 phosphorylation was delayed in tco2Δ cells. Both Tco1 and Tco2 contributed to fludioxonil sensitivity, with the double mutant displaying a similar level of resistance as hog1Δ cells and, consistent with the concept that Hog1 dephosphoryation accompanies stress resistance, Hog1 is trapped in its phosphorylated state in tco1Δtco2Δ cells. Regarding the stress-independent phenotypes under Hog1 regulation, tco1Δ cells are mating defective but display enhanced melanin production similar to Hog1 pathway mutants (Bahn et al., 2006). Thus, Tco2 appears to play a key role in relaying stress signals to Hog1 whereas Tco1 is the key sensor kinase in regulating melanin synthesis via the SAPK module. In this regard it is interesting that Tco1 and not Tco2 is important for virulence (Bahn et al., 2006). The functions of the remaining Tco3-7 sensor kinases remain to be determined.
Homologs of the S. cerevisiae Ypd1p phosphorelay protein and Ssk1p response regulator are present in C. neoformans. Ssk1 functions as a global regulator of the Hog1 pathway (Bahn et al., 2006). The high basal levels of Hog1 phosphorylation are dependent on Ssk1, suggesting that the polymorphisms in Ssk2 that drive Hog1 phosphorylation do not hyperactivate the MAPKKK per se, but promote a positive interaction with Ssk1 that enhances Ssk2 activity. Moreover, ssk1Δ mutants display overlapping phenotypes with those in the core SAPK module components, including impacted stress resistance, increased capsule and melanin production, and enhanced mating (Bahn et al., 2006). The interesting exception to this is responses to osmotic stress, as osmotic stress-induced dephosphorylation of Hog1 and resistance to this stress are Ssk1-independent (Bahn et al., 2006). This suggests the existence of an additional two-component independent pathway that relays osmotic stress signals to Hog1 but, in contrast to S. cerevisiae, this is not governed by the Sho1/Msb2 pathway (So et al., 2018). As in S. cerevisiae, deletion of the Ypd1 phosphorelay protein gives a lethal phenotype in C. neoformans and this is dependent on Hog1 presence (Lee et al., 2011). This suggests that, as in other fungi, Ypd1 negatively regulates Hog1 and thus loss of Ypd1 drives lethal levels of Hog1 phosphorylation in C. neoformans.
Regarding negative regulators of Hog1 phosphorylation, transcript profiling experiments in C. neoformans revealed that Hog1 was required for both basal levels and the stress induction of the protein tyrosine phosphatases PTP1 and PTP2 (Ko et al., 2009). A subsequent phenotypic analysis revealed Ptp2 to be a major regulator of Hog1, and Ptp2 but not Ptp1 is important for virulence (Lee et al., 2014a). Disruption of PTP2 increased the basal phosphorylation level of Hog1 and the stress induced dephosphorylation of Hog1 in response to osmotic stress was impaired in ptp2Δ cells. Cells lacking Ptp2 were actually more sensitive to osmotic stress than hog1Δ cells supporting the model that Ptp2-mediated dephosphorylation of Hog1 is needed to promote osmotic stress resistance (Lee et al., 2014a).
Concerning downstream responses of Hog1, transcript profiling analysis demonstrated that the SAPK module clearly regulates the C. neoformans transcriptome in the absence of stress with approximately 190 genes upregulated and 380 genes downregulated in hog1Δ cells compared to wild-type (Ko et al., 2009). Furthermore, a significant proportion of the deregulated genes were found to be Ssk1 dependent, consistent with previous work illustrating the importance of Ssk1 in Hog1 regulation (Bahn et al., 2006). Genes involved in melanin and capsule production, and those involved in the pheromone-Cpk1 MAPK pathway, were all upregulated in hog1Δ and ssk1Δ cells (Ko et al., 2009). This is highly consistent with the hyper-melanisation and encapsulation phenotypes, and enhanced mating exhibited by these mutants (Bahn et al., 2005, 2006). Interestingly, genes involved in ergosterol biosynthesis and resistance to heavy metals were upregulated in hog1Δ and ssk1Δ cells, promoting significantly higher ergosterol levels and cadmium resistance, respectively (Ko et al., 2009). The deregulation of ergosterol biosynthesis culminated with differential resistance profiles of Hog1 pathway mutants to the ergosterol-targeting antifungals, the triazoles and Amphotericin B; the increased induction of ERG11 likely contributes to the resistance of hog1Δ mutants to Erg11-targeting triazoles, whereas enhanced sterol levels probably explains the heightened sensitivity of Hog1 pathway mutants to the ergosterol-binding drug Amphotericin B (Ko et al., 2009). With regard to stress-induced gene expression a large number of osmotic and oxidative stress responsive genes were found to be dependent on the SAPK module in line with the stress-sensitive phenotypes exhibited by Hog1 pathway mutants. Subsequently, a number of studies have been published describing the functional analysis of a number of Hog1 target genes including the cation transporters Ena1 and Nha1 (Jung et al., 2012), the aquaporin Aqp1 (Meyers et al., 2017), the sulfiredoxin gene Srx1 (Upadhya et al., 2013), and the ferroxidases Cfo1 and Cfo2 (Lee et al., 2014b). Some of these targets undoubtedly contribute to the stress protective roles of the Hog1 pathway. For example, Hog1 is absolutely essential for the oxidative stress mediated induction of SRX1, and srx1Δ mutants are highly sensitive to H2O2 as Srx1 functions to reduce the thioredoxin peroxidase Tsa1, which becomes hyper-oxidized (inactivated) during peroxide detoxification (Upadhya et al., 2013). In a similar vein, Hog1 controls basal expression and osmotic stress induction of the cation transporters Ena1 and Nha1, and phenotypic characterization of ena1Δ and nha1Δ mutants indicates this likely contributes to osmotic and cationic stress resistance especially in glucose limiting environments (Ko et al., 2009; Jung et al., 2012).
A number of regulatory proteins that potentially function downstream of Hog1 in C. neoformans have also been identified. These include the Hrk1 and Sch9 kinases and the Atf1 and Mbs1 transcription factors. Hrk1 is orthologous to the MAPK-activated protein kinases, Rck2 in S. cerevisiae (Bilsland-Marchesan et al., 2000) and Srk1 in S. pombe (Smith et al., 2002), which are substrates for the respective Hog1 and Sty1 SAPKs. In C. neoformans HRK1 expression is Hog1 dependent and hog1Δ and hrx1Δ mutants display equivalent stress resistance to fludioxonil (Kim et al., 2011). Atf1 is orthologous to the S. pombe Atf1 transcription factor which is phosphorylated and regulated by the Sty1 SAPK (Lawrence et al., 2007). In C. neoformans Atf1 regulates some Hog1 dependent genes such as PTP1 and PTP3 (Lee et al., 2014a), and promotes resistance to several stresses (Missall and Lodge, 2005; Kim et al., 2010). However, whether Hrk1 or Atf1 is phosphorylated by Hog1 in C. neoformans is unknown, but the intriguing prediction based on stress-induced dephosphorylation of Hog1 is that phosphorylation of Hog1 substrates will be more dominant under non-stress conditions in this fungal pathogen.
Concluding Remarks
Although SAPKs are among the most evolutionarily conserved stress-signaling proteins in fungi (Nikolaou et al., 2009), their role and regulation has significantly diverged amongst fungal species. This review has highlighted how, in addition to stress resistance, SAPKs co-ordinate a myriad of distinct responses, dependant on the fungal pathogen in question. Considering regulation, in contrast to the dogma that stress-induced phosphorylation promotes SAPK-mediated stress resistance, in many C. neoformans species the Hog1 SAPK is constitutively phosphorylated and stress-induced dephosphorylation promotes stress resistance and stress-induced gene expression. Moreover, nitrosative stress triggers oxidation rather than stress-induced phosphorylation of C. albicans Hog1. A further noteworthy difference is that A. fumigatus has two highly related SAPK proteins and both must be deleted to fully prevent SAPK-mediated responses. Whilst the core SAPK module and downstream transcriptional responses are fairly well-characterized in the fungal pathogens considered in this review, major gaps in our knowledge remain. For example little is known regarding; (i) the signals sensed by the majority of the histidine kinases in C. albicans, A. fumigatus and C. neoformans, (ii) the mechanisms underlying two-component independent signaling to SAPKs, (iii) the downstream substrates phosphorylated by the active SAPK, and (iv) the precise role of these signaling modules in promoting virulence. It is important that such questions are addressed as SAPK pathways are important virulence attributes in many human, plant and insect infecting fungal pathogens (Brown et al., 2017). Thus, the development of drugs which target fungal SAPKs has the exciting potential to generate broad-acting antifungal treatments.
Author Contributions
JQ and AD contributed equally to the writing of this review.
Funding
Work in the JQ laboratory is funded by the BBSRC (BB/K016939/1, BB/P020119/1).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aguilera, J., Rodriguez-Vargas, S., and Prieto, J. A. (2005). The HOG MAP kinase pathway is required for the induction of methylglyoxal-responsive genes and determines methylglyoxal resistance in Saccharomyces cerevisiae. Mol. Microbiol. 56, 228–239. doi: 10.1111/j.1365-2958.2005.04533.x
Alonso-Monge, R., Carvaihlo, S., Nombela, C., Rial, E., and Pla, J. (2009). The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 155(Pt 2), 413–423. doi: 10.1099/mic.0.023309-0
Alonso-Monge, R., Navarro-Garcia, F., Molero, G., Diez-Orejas, R., Gustin, M., Pla, J., et al. (1999). Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181, 3058–3068.
Alonso-Monge, R., Navarro-Garcia, F., Roman, E., Negredo, A. I., Eisman, B., Nombela, C., et al. (2003). The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryotic Cell 2, 351–361. doi: 10.1128/EC.2.2.351-361.2003
Altwasser, R., Baldin, C., Weber, J., Guthke, R., Kniemeyer, O., Brakhage, A. A., et al. (2015). Network modeling reveals cross talk of MAP kinases during adaptation to caspofungin stress in Aspergillus fumigatus. PLoS ONE 10:e0136932. doi: 10.1371/journal.pone.0136932
Arana, D. M., Alonso-Monge, R., Du, C., Calderone, R., and Pla, J. (2007). Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell. Microbiol. 9, 1647–1659. doi: 10.1111/j.1462-5822.2007.00898.x
Arana, D. M., Nombela, C., Alonso-Monge, R., and Pla, J. (2005). The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151(Pt 4), 1033–1049. doi: 10.1099/mic.0.27723-0
Argimon, S., Fanning, S., Blankenship, J. R., and Mitchell, A. P. (2011). Interaction between the Candida albicans high-osmolarity glycerol (HOG) pathway and the response to human beta-defensins 2 and 3. Eukaryotic Cell 10, 272–275. doi: 10.1128/EC.00133-10
Bahn, Y. S. (2008). Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryotic Cell 7, 2017–2036. doi: 10.1128/EC.00323-08
Bahn, Y. S., Geunes-Boyer, S., and Heitman, J. (2007). Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryotic Cell 6, 2278–2289. doi: 10.1128/EC.00349-07
Bahn, Y. S., Kojima, K., Cox, G. M., and Heitman, J. (2005). Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16, 2285–2300. doi: 10.1091/mbc.e04-11-0987
Bahn, Y. S., Kojima, K., Cox, G. M., and Heitman, J. (2006). A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 17, 3122–3135. doi: 10.1091/mbc.e06-02-0113
Banerjee, D., Bloom, A. L., and Panepinto, J. C. (2016). Opposing PKA and Hog1 signals control the post-transcriptional response to glucose availability in Cryptococcus neoformans. Mol. Microbiol. 102, 306–320. doi: 10.1111/mmi.13461
Bilsland, E., Molin, C., Swaminathan, S., Ramne, A., and Sunnerhagen, P. (2004). Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 53, 1743–1756. doi: 10.1111/j.1365-2958.2004.04238.x
Bilsland-Marchesan, E., Arino, J., Saito, H., Sunnerhagen, P., and Posas, F. (2000). Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 20, 3887–3895. doi: 10.1128/MCB.20.11.3887-3895.2000
Brewster, J. L., de Valoir, T., Dwyer, N. D., Winter, E., and Gustin, M. C. (1993). An osmosensing signal transduction pathway in yeast. Science 259, 1760–1763. doi: 10.1126/science.7681220
Brewster, J. L., and Gustin, M. C. (2014). Hog1: 20 years of discovery and impact. Sci. Signal. 7:re7. doi: 10.1126/scisignal.2005458
Brown, A. J. P., Cowen, L. E., di Pietro, A., and Quinn, J. (2017). Stress adaptation. Microbiol. Spectr. 5. doi: 10.1128/microbiolspec.FUNK-0048-2016
Brown, G. D., Denning, D. W., Gow, N. A., Levitz, S. M., Netea, M. G., and White, T. C. (2012). Hidden killers: human fungal infections. Sci. Transl. Med. 4:165rv113. doi: 10.1126/scitranslmed.3004404
Bruder Nascimento, A. C., Dos Reis, T. F., de Castro, P. A., Hori, J. I., Bom, V. L., de Assis, L. J., et al. (2016). Mitogen activated protein kinases SakA(HOG1) and MpkC collaborate for Aspergillus fumigatus virulence. Mol. Microbiol. 100, 841–859. doi: 10.1111/mmi.13354
Buck, V., Quinn, J., Soto Pino, T., Martin, H., Saldanha, J., Makino, K., et al. (2001). Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12, 407–419. doi: 10.1091/mbc.12.2.407
Buschart, A., Gremmer, K., El-Mowafy, M., van den Heuvel, J., Mueller, P. P., and Bilitewski, U. (2012). A novel functional assay for fungal histidine kinases group III reveals the role of HAMP domains for fungicide sensitivity. J. Biotechnol. 157, 268–277. doi: 10.1016/j.jbiotec.2011.09.017
Calera, J. A., Zhao, X. J., and Calderone, R. (2000). Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun. 68, 518–525. doi: 10.1128/IAI.68.2.518-525.2000
Calera, J. A., Zhao, X. J., De Bernardis, F., Sheridan, M., and Calderone, R. (1999). Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect. Immun. 67, 4280–4284.
Chapeland-Leclerc, F., Dilmaghani, A., Ez-Zaki, L., Boisnard, S., Da Silva, B., Gaslonde, T., et al. (2015). Systematic gene deletion and functional characterization of histidine kinase phosphorelay receptors (HKRs) in the human pathogenic fungus Aspergillus fumigatus. Fungal Genet. Biol. 84, 1–11. doi: 10.1016/j.fgb.2015.09.005
Chauhan, N., Inglis, D., Roman, E., Pla, J., Li, D., Calera, J. A., et al. (2003). Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryotic Cell 2, 1018–1024. doi: 10.1128/EC.2.5.1018-1024.2003
Cheetham, J., MacCallum, D. M., Doris, K. S., da Silva Dantas, A., Scorfield, S., Odds, F., et al. (2011). MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J. Biol. Chem. 286, 42002–42016. doi: 10.1074/jbc.M111.265231
Cheetham, J., Smith, D. A., da Silva Dantas, A., Doris, K. S., Patterson, M. J., Bruce, C. R., et al. (2007). A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol. Biol. Cell 18, 4603–4614. doi: 10.1091/mbc.e07-06-0581
Chien, C. T., Chen, Y. C., Liu, Y. C., Liang, S. H., Lin, H. H., and Lin, C. H. (2018). The antimicrobial photodynamic inactivation resistance of Candida albicans is modulated by the Hog1 pathway and the Cap1 transcription factor. Med. Mycol. doi: 10.1093/mmy/myy079. [Epub ahead of print].
Clemons, K. V., Miller, T. K., Selitrennikoff, C. P., and Stevens, D. A. (2002). fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med. Mycol. 40, 259–262. doi: 10.1080/mmy.40.3.259.262
Clotet, J., and Posas, F. (2007). Control of cell cycle in response to osmostress: lessons from yeast. Meth. Enzymol. 428, 63–76. doi: 10.1016/S0076-6879(07)28004-8
Correia, I., Alonso-Monge, R., and Pla, J. (2016). The Hog1 MAP kinase promotes the recovery from cell cycle arrest induced by hydrogen peroxide in Candida albicans. Front. Microbiol. 7:2133. doi: 10.3389/fmicb.2016.02133
da Silva Dantas, A., Patterson, M. J., Smith, D. A., Maccallum, D. M., Erwig, L. P., Morgan, B. A., et al. (2010). Thioredoxin regulates multiple hydrogen peroxide-induced signaling pathways in Candida albicans. Mol. Cell. Biol. 30, 4550–4563. doi: 10.1128/MCB.00313-10
Day, A. M., Herrero-de-Dios, C. M., MacCallum, D. M., Brown, A. J. P., and Quinn, J. (2017a). Stress-induced nuclear accumulation is dispensable for Hog1-dependent gene expression and virulence in a fungal pathogen. Sci. Rep. 7:14340. doi: 10.1038/s41598-017-14756-4
Day, A. M., Smith, D. A., Ikeh, M. A., Haider, M., Herrero-de-Dios, C. M., Brown, A. J., et al. (2017b). Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation. PLoS Pathog. 13:e1006131. doi: 10.1371/journal.ppat.1006131
de Assis, L. J., Manfiolli, A., Mattos, E., Fabri, J., Malavazi, I., Jacobsen, I. D., et al. (2018). Protein kinase a and high-osmolarity glycerol response pathways cooperatively control cell wall carbohydrate mobilization in Aspergillus fumigatus. MBio 9:e01952–18. doi: 10.1128/mBio.01952-18
de Castro, P. A., Chen, C., de Almeida, R. S., Freitas, F. Z., Bertolini, M. C., Morais, E. R., et al. (2014). ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Mol. Microbiol. 94, 655–674. doi: 10.1111/mmi.12785
de Nadal, E., and Posas, F. (2010). Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 29, 4–13. doi: 10.1038/emboj.2009.346
Dihazi, H., Kessler, R., and Eschrich, K. (2004). High osmolarity glycerol (HOG) pathway-induced phosphorylation and activation of 6-phosphofructo-2-kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. J. Biol. Chem. 279, 23961–23968. doi: 10.1074/jbc.M312974200
Du, C., Sarfati, J., Latge, J. P., and Calderone, R. (2006). The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med. Mycol. 44, 211–218. doi: 10.1080/13693780500338886
Egger, L. A., Park, H., and Inouye, M. (1997). Signal transduction via the histidyl-aspartyl phosphorelay. Genes Cells 2, 167–184. doi: 10.1046/j.1365-2443.1997.d01-311.x
Eisman, B., Alonso-Monge, R., Roman, E., Arana, D., Nombela, C., and Pla, J. (2006). The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryotic Cell 5, 347–358. doi: 10.1128/EC.5.2.347-358.2006
Enjalbert, B., Smith, D. A., Cornell, M. J., Alam, I., Nicholls, S., Brown, A. J., et al. (2006). Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell 17, 1018–1032. doi: 10.1091/mbc.e05-06-0501
Fabri, J., Godoy, N. L., Rocha, M. C., Munshi, M., Cocio, T. A., von Zeska Kress, M. R., et al. (2018). The AGC kinase YpkA regulates sphingolipids biosynthesis and physically interacts with SakA MAP kinase in Aspergillus fumigatus. Front. Microbiol. 9:3347. doi: 10.3389/fmicb.2018.03347
Ferrigno, P., Posas, F., Koepp, D., Saito, H., and Silver, P. A. (1998). Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17, 5606–5614. doi: 10.1093/emboj/17.19.5606
Fuller, K. K., Ringelberg, C. S., Loros, J. J., and Dunlap, J. C. (2013). The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. MBio 4:e00142–13. doi: 10.1128/mBio.00142-13
Galcheva-Gargova, Z., Derijard, B., Wu, I. H., and Davis, R. J. (1994). An osmosensing signal transduction pathway in mammalian cells. Science 265, 806–808. doi: 10.1126/science.8047888
Hagen, F., Khayhan, K., Theelen, B., Kolecka, A., Polacheck, I., Sionov, E., et al. (2015). Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 78, 16–48. doi: 10.1016/j.fgb.2015.02.009
Hagiwara, D., Suzuki, S., Kamei, K., Gonoi, T., and Kawamoto, S. (2014). The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet. Biol. 73, 138–149. doi: 10.1016/j.fgb.2014.10.011
Hagiwara, D., Takahashi-Nakaguchi, A., Toyotome, T., Yoshimi, A., Abe, K., Kamei, K., et al. (2013). NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS ONE 8:e80881. doi: 10.1371/journal.pone.0080881
Han, J., Lee, J. D., Bibbs, L., and Ulevitch, R. J. (1994). A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811. doi: 10.1126/science.7914033
Hayashi, M., and Maeda, T. (2006). Activation of the HOG pathway upon cold stress in Saccharomyces cerevisiae. J. Biochem. 139, 797–803. doi: 10.1093/jb/mvj089
Hayes, B. M., Bleackley, M. R., Wiltshire, J. L., Anderson, M. A., Traven, A., and van der Weerden, N. L. (2013). Identification and mechanism of action of the plant defensin NaD1 as a new member of the antifungal drug arsenal against Candida albicans. Antimicrob. Agents Chemother. 57, 3667–3675. doi: 10.1128/AAC.00365-13
Herrero-de-Dios, C., Day, A. M., Tillmann, A. T., Kastora, S. L., Stead, D., Salgado, P. S., et al. (2018). Redox regulation, rather than stress-induced phosphorylation, of a Hog1 mitogen-activated protein kinase modulates its nitrosative-stress-specific outputs. MBio 9:e02229–17. doi: 10.1128/mBio.02229-17
Hickman, M. J., Spatt, D., and Winston, F. (2011). The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188, 325–338. doi: 10.1534/genetics.111.128322
Hopke, A., Nicke, N., Hidu, E. E., Degani, G., Popolo, L., and Wheeler, R. T. (2016). Neutrophil attack triggers extracellular trap-dependent candida cell wall remodeling and altered immune recognition. PLoS Pathog. 12:e1005644. doi: 10.1371/journal.ppat.1005644
Ji, Y., Yang, F., Ma, D., Zhang, J., Wan, Z., Liu, W., et al. (2012). HOG-MAPK signaling regulates the adaptive responses of Aspergillus fumigatus to thermal stress and other related stress. Mycopathologia 174, 273–282. doi: 10.1007/s11046-012-9557-4
Jung, K. W., Strain, A. K., Nielsen, K., Jung, K. H., and Bahn, Y. S. (2012). Two cation transporters Ena1 and Nha1 cooperatively modulate ion homeostasis, antifungal drug resistance, and virulence of Cryptococcus neoformans via the HOG pathway. Fungal Genet. Biol. 49, 332–345. doi: 10.1016/j.fgb.2012.02.001
Kaba, H. E., Nimtz, M., Muller, P. P., and Bilitewski, U. (2013). Involvement of the mitogen activated protein kinase Hog1p in the response of Candida albicans to iron availability. BMC Microbiol. 13:16. doi: 10.1186/1471-2180-13-16
Kelly, J., Rowan, R., McCann, M., and Kavanagh, K. (2009). Exposure to caspofungin activates Cap and Hog pathways in Candida albicans. Med. Mycol. 47, 697–706. doi: 10.3109/13693780802552606
Kim, J. H., Chan, K. L., Faria, N. C., Martins Mde, L., and Campbell, B. C. (2012). Targeting the oxidative stress response system of fungi with redox-potent chemosensitizing agents. Front. Microbiol. 3:88. doi: 10.3389/fmicb.2012.00088
Kim, M. S., Ko, Y. J., Maeng, S., Floyd, A., Heitman, J., and Bahn, Y. S. (2010). Comparative transcriptome analysis of the CO2 sensing pathway via differential expression of carbonic anhydrase in Cryptococcus neoformans. Genetics 185, 1207–1219. doi: 10.1534/genetics.110.118315
Kim, S. Y., Ko, Y. J., Jung, K. W., Strain, A., Nielsen, K., and Bahn, Y. S. (2011). Hrk1 plays both Hog1-dependent and -independent roles in controlling stress response and antifungal drug resistance in Cryptococcus neoformans. PLoS ONE 6:e18769. doi: 10.1371/journal.pone.0018769
Ko, Y. J., Yu, Y. M., Kim, G. B., Lee, G. W., Maeng, P. J., Kim, S., et al. (2009). Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryotic Cell 8, 1197–1217. doi: 10.1128/EC.00120-09
Kojima, K., Takano, Y., Yoshimi, A., Tanaka, C., Kikuchi, T., and Okuno, T. (2004). Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 53, 1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x
Lawrence, C. L., Maekawa, H., Worthington, J. L., Reiter, W., Wilkinson, C. R., and Jones, N. (2007). Regulation of Schizosaccharomyces pombe Atf1 protein levels by Sty1-mediated phosphorylation and heterodimerization with Pcr1. J. Biol. Chem. 282, 5160–5170. doi: 10.1074/jbc.M608526200
Lee, J., and Levin, D. E. (2018). Intracellular mechanism by which arsenite activates the yeast stress MAPK Hog1. Mol. Biol. Cell 29, 1904–1915. doi: 10.1091/mbc.E18-03-0185
Lee, J., Reiter, W., Dohnal, I., Gregori, C., Beese-Sims, S., Kuchler, K., et al. (2013). MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev. 27, 2590–2601. doi: 10.1101/gad.229310.113
Lee, J. W., Ko, Y. J., Kim, S. Y., and Bahn, Y. S. (2011). Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryotic Cell 10, 998–1002. doi: 10.1128/EC.05124-11
Lee, K. T., Byun, H. J., Jung, K. W., Hong, J., Cheong, E., and Bahn, Y. S. (2014a). Distinct and redundant roles of protein tyrosine phosphatases Ptp1 and Ptp2 in governing the differentiation and pathogenicity of Cryptococcus neoformans. Eukaryotic Cell 13, 796–812. doi: 10.1128/EC.00069-14
Lee, K. T., Lee, J. W., Lee, D., Jung, W. H., and Bahn, Y. S. (2014b). A ferroxidase, Cfo1, regulates diverse environmental stress responses of Cryptococcus neoformans through the HOG pathway. Mycobiology 42, 152–157. doi: 10.5941/MYCO.2014.42.2.152
Li, D., Gurkovska, V., Sheridan, M., Calderone, R., and Chauhan, N. (2004). Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology 150(Pt 10), 3305–3313. doi: 10.1099/mic.0.27237-0
Li, X., Huang, X., Zhao, J., Zhao, J., Wei, Y., and Jiang, L. (2008). The MAP kinase-activated protein kinase Rck2p plays a role in rapamycin sensitivity in Saccharomyces cerevisiae and Candida albicans. FEMS Yeast Res. 8, 715–724. doi: 10.1111/j.1567-1364.2008.00402.x
Liang, S. H., Cheng, J. H., Deng, F. S., Tsai, P. A., and Lin, C. H. (2014). A novel function for Hog1 stress-activated protein kinase in controlling white-opaque switching and mating in Candida albicans. Eukaryotic Cell 13, 1557–1566. doi: 10.1128/EC.00235-14
Lo, H. J., Kohler, J. R., DiDomenico, B., Loebenberg, D., Cacciapuoti, A., and Fink, G. R. (1997). Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939–949. doi: 10.1016/S0092-8674(00)80358-X
Maeda, T., Wurgler-Murphy, S. M., and Saito, H. (1994). A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369, 242–245. doi: 10.1038/369242a0
Marotta, D. H., Nantel, A., Sukala, L., Teubl, J. R., and Rauceo, J. M. (2013). Genome-wide transcriptional profiling and enrichment mapping reveal divergent and conserved roles of Sko1 in the Candida albicans osmotic stress response. Genomics 102, 363–371. doi: 10.1016/j.ygeno.2013.06.002
Mavrianos, J., Desai, C., and Chauhan, N. (2014). Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans. Eukaryotic Cell 13, 452–460. doi: 10.1128/EC.00243-13
McCormick, A., Jacobsen, I. D., Broniszewska, M., Beck, J., Heesemann, J., and Ebel, F. (2012). The two-component sensor kinase TcsC and its role in stress resistance of the human-pathogenic mold Aspergillus fumigatus. PLoS ONE 7:e38262. doi: 10.1371/journal.pone.0038262
Meyers, G. L., Jung, K. W., Bang, S., Kim, J., Kim, S., Hong, J., et al. (2017). The water channel protein aquaporin 1 regulates cellular metabolism and competitive fitness in a global fungal pathogen Cryptococcus neoformans. Environ. Microbiol. Rep. 9, 268–278. doi: 10.1111/1758-2229.12527
Missall, T. A., and Lodge, J. K. (2005). Function of the thioredoxin proteins in Cryptococcus neoformans during stress or virulence and regulation by putative transcriptional modulators. Mol. Microbiol. 57, 847–858. doi: 10.1111/j.1365-2958.2005.04735.x
Mollapour, M., and Piper, P. W. (2007). Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol. Cell. Biol. 27, 6446–6456. doi: 10.1128/MCB.02205-06
Nagahashi, S., Mio, T., Ono, N., Yamada-Okabe, T., Arisawa, M., Bussey, H., et al. (1998). Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 144 (Pt 2), 425–432. doi: 10.1099/00221287-144-2-425
Navarro-Garcia, F., Eisman, B., Fiuza, S. M., Nombela, C., and Pla, J. (2005). The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 151(Pt 8), 2737–2749. doi: 10.1099/mic.0.28038-0
Nicholls, S., Straffon, M., Enjalbert, B., Nantel, A., Macaskill, S., Whiteway, M., et al. (2004). Msn2- and Msn4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen Candida albicans. Eukaryotic Cell 3, 1111–1123. doi: 10.1128/EC.3.5.1111-1123.2004
Nikolaou, E., Agrafioti, I., Stumpf, M., Quinn, J., Stansfield, I., and Brown, A. J. (2009). Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol. Biol. 9:44. doi: 10.1186/1471-2148-9-44
O'Meara, T. R., Duah, K., Guo, C. X., Maxson, M. E., Gaudet, R. G., Koselny, K., et al. (2018). High-throughput screening identifies genes required for Candida albicans induction of macrophage pyroptosis. MBio 9:e01581–18. doi: 10.1128/mBio.01581-18
Pascual-Ahuir, A., and Proft, M. (2007). The Sch9 kinase is a chromatin-associated transcriptional activator of osmostress-responsive genes. EMBO J. 26, 3098–3108. doi: 10.1038/sj.emboj.7601756
Pereira Silva, L., Alves de Castro, P., Dos Reis, T. F., Paziani, M. H., Von Zeska Kress, M. R., Riano-Pachon, D. M., et al. (2017). Genome-wide transcriptome analysis of Aspergillus fumigatus exposed to osmotic stress reveals regulators of osmotic and cell wall stresses that are SakA(HOG1) and MpkC dependent. Cell. Microbiol. 19:e12681. doi: 10.1111/cmi.12681
Polvi, E. J., Averette, A. F., Lee, S. C., Kim, T., Bahn, Y. S., Veri, A. O., et al. (2016). Metal chelation as a powerful strategy to probe cellular circuitry governing fungal drug resistance and morphogenesis. PLoS Genet. 12:e1006350. doi: 10.1371/journal.pgen.1006350
Posas, F., and Saito, H. (1997). Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science 276, 1702–1705. doi: 10.1126/science.276.5319.1702
Posas, F., and Saito, H. (1998). Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17, 1385–1394. doi: 10.1093/emboj/17.5.1385
Posas, F., Wurgler-Murphy, S. M., Maeda, T., Witten, E. A., Thai, T. C., and Saito, H. (1996). Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86, 865–875. doi: 10.1016/S0092-8674(00)80162-2
Pott, G. B., Miller, T. K., Bartlett, J. A., Palas, J. S., and Selitrennikoff, C. P. (2000). The isolation of FOS-1, a gene encoding a putative two-component histidine kinase from Aspergillus fumigatus. Fungal Genet. Biol. 31, 55–67. doi: 10.1006/fgbi.2000.1225
Prieto, D., Roman, E., Correia, I., and Pla, J. (2014). The HOG pathway is critical for the colonization of the mouse gastrointestinal tract by Candida albicans. PLoS ONE 9:e87128. doi: 10.1371/journal.pone.0087128
Proft, M., and Struhl, K. (2002). Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9, 1307–1317. doi: 10.1016/S1097-2765(02)00557-9
Rajasingham, R., Smith, R. M., Park, B. J., Jarvis, J. N., Govender, N. P., Chiller, T. M., et al. (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17, 873–881. doi: 10.1016/S1473-3099(17)30243-8
Rauceo, J. M., Blankenship, J. R., Fanning, S., Hamaker, J. J., Deneault, J. S., Smith, F. J., et al. (2008). Regulation of the Candida albicans cell wall damage response by transcription factor Sko1 and PAS kinase Psk1. Mol. Biol. Cell 19, 2741–2751. doi: 10.1091/mbc.e08-02-0191
Reyes, G., Romans, A., Nguyen, C. K., and May, G. S. (2006). Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryotic Cell 5, 1934–1940. doi: 10.1128/EC.00178-06
Rodaki, A., Bohovych, I. M., Enjalbert, B., Young, T., Odds, F. C., Gow, N. A., et al. (2009). Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol. Biol. Cell 20, 4845–4855. doi: 10.1091/mbc.e09-01-0002
Roman, E., Nombela, C., and Pla, J. (2005). The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol. Cell. Biol. 25, 10611–10627. doi: 10.1128/MCB.25.23.10611-10627.2005
Saito, H., and Posas, F. (2012). Response to hyperosmotic stress. Genetics 192, 289–318. doi: 10.1534/genetics.112.140863
Salas-Delgado, G., Ongay-Larios, L., Kawasaki-Watanabe, L., Lopez-Villasenor, I., and Coria, R. (2017). The yeasts phosphorelay systems: a comparative view. World J. Microbiol. Biotechnol. 33:111. doi: 10.1007/s11274-017-2272-z
San Jose, C., Monge, R. A., Perez-Diaz, R., Pla, J., and Nombela, C. (1996). The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178, 5850–5852. doi: 10.1128/jb.178.19.5850-5852.1996
Selitrennikoff, C. P., Alex, L., Miller, T. K., Clemons, K. V., Simon, M. I., and Stevens, D. A. (2001). COS-l, a putative two-component histidine kinase of Candida albicans, is an in vivo virulence factor. Med. Mycol. 39, 69–74. doi: 10.1080/mmy.39.1.69.74
Sellam, A., Chaillot, J., Mallick, J., Tebbji, F., Richard Albert, J., Cook, M. A., et al. (2019). The p38/HOG stress-activated protein kinase network couples growth to division in Candida albicans. PLoS Genet. 15:e1008052. doi: 10.1371/journal.pgen.1008052
Shor, E., and Chauhan, N. (2015). A case for two-component signaling systems as antifungal drug targets. PLoS Pathog. 11:e1004632. doi: 10.1371/journal.ppat.1004632
Smith, D. A., Morgan, B. A., and Quinn, J. (2010). Stress signalling to fungal stress-activated protein kinase pathways. FEMS Microbiol. Lett. 306, 1–8. doi: 10.1111/j.1574-6968.2010.01937.x
Smith, D. A., Nicholls, S., Morgan, B. A., Brown, A. J., and Quinn, J. (2004). A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell 15, 4179–4190. doi: 10.1091/mbc.e04-03-0181
Smith, D. A., Toone, W. M., Chen, D., Bahler, J., Jones, N., Morgan, B. A., et al. (2002). The Srk1 protein kinase is a target for the Sty1 stress-activated MAPK in fission yeast. J. Biol. Chem. 277, 33411–33421. doi: 10.1074/jbc.M204593200
So, Y. S., Jang, J., Park, G., Xu, J., Olszewski, M. A., and Bahn, Y. S. (2018). Sho1 and Msb2 play complementary but distinct roles in stress responses, sexual differentiation, and pathogenicity of Cryptococcus neoformans. Front. Microbiol. 9:2958. doi: 10.3389/fmicb.2018.02958
Su, C., Lu, Y., and Liu, H. (2013). Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol. Biol. Cell 24, 385–397. doi: 10.1091/mbc.e12-06-0477
Tanaka, K., Tatebayashi, K., Nishimura, A., Yamamoto, K., Yang, H. Y., and Saito, H. (2014). Yeast osmosensors Hkr1 and Msb2 activate the Hog1 MAPK cascade by different mechanisms. Sci. Signal. 7:ra21. doi: 10.1126/scisignal.2004780
Tatebayashi, K., Takekawa, M., and Saito, H. (2003). A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway. EMBO J. 22, 3624–3634. doi: 10.1093/emboj/cdg353
Tatebayashi, K., Yamamoto, K., Nagoya, M., Takayama, T., Nishimura, A., Sakurai, M., et al. (2015). Osmosensing and scaffolding functions of the oligomeric four-transmembrane domain osmosensor Sho1. Nat. Commun. 6:6975. doi: 10.1038/ncomms7975
Tatebayashi, K., Yamamoto, K., Tanaka, K., Tomida, T., Maruoka, T., Kasukawa, E., et al. (2006). Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J. 25, 3033–3044. doi: 10.1038/sj.emboj.7601192
Thomas, E., Roman, E., Claypool, S., Manzoor, N., Pla, J., and Panwar, S. L. (2013). Mitochondria influence CDR1 efflux pump activity, Hog1-mediated oxidative stress pathway, iron homeostasis, and ergosterol levels in Candida albicans. Antimicrob. Agents Chemother. 57, 5580–5599. doi: 10.1128/AAC.00889-13
Torosantucci, A., Chiani, P., De Bernardis, F., Cassone, A., Calera, J. A., and Calderone, R. (2002). Deletion of the two-component histidine kinase gene (CHK1) of Candida albicans contributes to enhanced growth inhibition and killing by human neutrophils in vitro. Infect. Immun. 70, 985–987. doi: 10.1128/IAI.70.2.985-987.2002
Upadhya, R., Kim, H., Jung, K. W., Park, G., Lam, W., Lodge, J. K., et al. (2013). Sulphiredoxin plays peroxiredoxin-dependent and -independent roles via the HOG signalling pathway in Cryptococcus neoformans and contributes to fungal virulence. Mol. Microbiol. 90, 630–648. doi: 10.1111/mmi.12388
Veal, E. A., Findlay, V. J., Day, A. M., Bozonet, S. M., Evans, J. M., Quinn, J., et al. (2004). A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol. Cell 15, 129–139. doi: 10.1016/j.molcel.2004.06.021
Vendrell, A., Martinez-Pastor, M., Gonzalez-Novo, A., Pascual-Ahuir, A., Sinclair, D. A., Proft, M., et al. (2011). Sir2 histone deacetylase prevents programmed cell death caused by sustained activation of the Hog1 stress-activated protein kinase. EMBO Rep. 12, 1062–1068. doi: 10.1038/embor.2011.154
Vylkova, S., Jang, W. S., Li, W., Nayyar, N., and Edgerton, M. (2007). Histatin 5 initiates osmotic stress response in Candida albicans via activation of the Hog1 mitogen-activated protein kinase pathway. Eukaryotic Cell 6, 1876–1888. doi: 10.1128/EC.00039-07
Warmka, J., Hanneman, J., Lee, J., Amin, D., and Ota, I. (2001). Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 21, 51–60. doi: 10.1128/MCB.21.1.51-60.2001
Westfall, P. J., Patterson, J. C., Chen, R. E., and Thorner, J. (2008). Stress resistance and signal fidelity independent of nuclear MAPK function. Proc. Natl. Acad. Sci. U.S.A. 105, 12212–12217. doi: 10.1073/pnas.0805797105
Winkelstroter, L. K., Bom, V. L., de Castro, P. A., Ramalho, L. N., Goldman, M. H., Brown, N. A., et al. (2015). High osmolarity glycerol response PtcB phosphatase is important for Aspergillus fumigatus virulence. Mol. Microbiol. 96, 42–54. doi: 10.1111/mmi.12919
Winkler, A., Arkind, C., Mattison, C. P., Burkholder, A., Knoche, K., and Ota, I. (2002). Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryotic Cell 1, 163–173. doi: 10.1128/EC.1.2.163-173.2002
Wong Sak Hoi, J., Beau, R., and Latge, J. P. (2012). A novel dehydrin-like protein from Aspergillus fumigatus regulates freezing tolerance. Fungal Genet. Biol. 49, 210–216. doi: 10.1016/j.fgb.2012.01.005
Wurgler-Murphy, S. M., Maeda, T., Witten, E. A., and Saito, H. (1997). Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 17, 1289–1297. doi: 10.1128/MCB.17.3.1289
Yamada-Okabe, T., Mio, T., Ono, N., Kashima, Y., Matsui, M., Arisawa, M., et al. (1999). Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181, 7243–7247.
Keywords: Hog1, Cryptococcus, Candida albicans, Aspergillus fumigatus, SAPK, stress signaling, fungal pathogenesis
Citation: Day AM and Quinn J (2019) Stress-Activated Protein Kinases in Human Fungal Pathogens. Front. Cell. Infect. Microbiol. 9:261. doi: 10.3389/fcimb.2019.00261
Received: 09 May 2019; Accepted: 04 July 2019;
Published: 17 July 2019.
Edited by:
Yong-Sun Bahn, Yonsei University, South KoreaReviewed by:
Won Hee Jung, Chung-Ang University, South KoreaMichael S. Price, Liberty University, United States
Copyright © 2019 Day and Quinn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison M. Day, a.m.day@ncl.ac.uk; Janet Quinn, janet.quinn@ncl.ac.uk
 Alison M. Day*
Alison M. Day*  Janet Quinn
Janet Quinn