- 1Institute of Cotton, Anhui Academy of Agricultural Sciences, Hefei, China
- 2Key Laboratory of Jiangxi Province for Environment and Energy Catalysis, Institute of Applied Chemistry, School of Chemistry and Chemical Engineering, Nanchang University, Nanchang, China
- 3Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, China
- 4Department of Chemical and Process Engineering, University of Surrey, Guildford, United Kingdom
- 5Department of Inorganic Chemistry and Materials Sciences Institute, University of Seville-CSIC, Seville, Spain
The rapid economic and societal development have led to unprecedented energy demand and consumption resulting in the harmful emission of pollutants. Hence, the conversion of greenhouse gases into valuable chemicals and fuels has become an urgent challenge for the scientific community. In recent decades, perovskite-type mixed oxide-based catalysts have attracted significant attention as efficient CO2 conversion catalysts due to the characteristics of both reversible oxygen storage capacity and stable structure compared to traditional oxide-supported catalysts. In this review, we hand over a comprehensive overview of the research for CO2 conversion by these emerging perovskite-type mixed oxide-based catalysts. Three main CO2 conversions, namely reverse water gas shift reaction, CO2 methanation, and CO2 reforming of methane have been introduced over perovskite-type mixed oxide-based catalysts and their reaction mechanisms. Different approaches for promoting activity and resisting carbon deposition have also been discussed, involving increased oxygen vacancies, enhanced dispersion of active metal, and fine-tuning strong metal-support interactions. Finally, the current challenges are mooted, and we have proposed future research prospects in this field to inspire more sensational breakthroughs in the material and environment fields.
Introduction
The rapid development of society and the economy has led to the huge demand for global energy (Vignieri, 2020). Although renewable energy resources such as tidal, geothermal power, wind, and solar have emerged in recent years, traditional fossil fuels including coal, oil, and natural gas are still dominant within the energy portfolio (Li 2021; Zhao et al., 2021). The high reliance on fossil fuels is accompanied by massive greenhouse gases (GHGs) emissions, mostly in the form of carbon dioxide (CO2), which brews a potential threat to the ecological environment and human health (Roy et al., 2018). According to the World Energy Statistical Yearbook (70th Edition) released by the British Petroleum Company, global carbon emissions have maintained continuous growth since 2013 and reached a formidable record of 3.436 × 1010 tons in 2019 (Hu et al., 2021). As a result, a series of global action plans such as the Intergovernmental Panel on Climate Change (IPCC), the United Nations Climate Change Conference (COP21, Paris, 2015), and the International Energy Agency (IEA) have accentuated the imperativeness to diminish CO2 emissions by at least half of the current amount by 2050 (Roy et al., 2018; Hussain et al., 2021). China has come up with the target to reach a “carbon peak” by 2030 and be “carbon neutral” by 2060 in carbon dioxide emissions (Wang et al., 2020; Li 2021; Zhao et al., 2021). Therefore, the conversion and utilization of waste CO2 emissions into higher-value commodities while mitigating climate change has drawn great attention, which is critical for a sustainable future (Ye et al., 2019a; Sun et al., 2020; Ye et al., 2020).
However, CO2 is a highly oxidized, thermodynamically stable molecule (∆G0 = -400 kJ/mol) with ultra-low reactivity, which requires surmounting the tremendous thermodynamic activation barrier. Thus, the chemical conversion and economic utilization of CO2 is an awesome scientific and technical challenge (Ashok et al., 2020). CO2 is mainly used as raw material to manufacture fuels or bulk chemicals for the chemical industry in the following ways: 1) CO2 to CO (Chen et al., 2020; Kopac et al., 2020; Lim et al., 2021b); 2) CO2 to CH4 (Shin et al., 2016; Ulmer et al., 2019; Wang X et al., 2019); 3) CO2 to CH3OH (Zhan et al., 2014; Li et al., 2017; Li F et al., 2019); 4) CO2 to bulk chemicals like DME, urea, salicylic acid, polycarbonates (Utsis et al., 2016; Ye et al., 2019a; An et al., 2021). Among the proposed CO2 recycling options, catalytic CO2 hydrogenation to carbon fuels, especially via CO as an intermediate for the Fischer-Tropsch synthesis to generate more complicated chemicals, is of particular industrial importance (Gao et al., 2020). Thence, hydrogenation reaction has been regarded as an influential chemical conversion of CO2 since it offers a promising prospect to achieve sustainable development in energy and the environment. However, CO2 hydrogenation and conversion technology are still challenging for inadequate conversion and poor selectivity, which are outcomes of unfavorable kinetic and thermodynamic factors (Moradi et al., 2010). For example, CO2 conversion involves selective reduction of CO2 with H2 or another reductant under high temperatures and pressures, while metal-based catalysts used are inclined to sinter and deactivate under severe operating conditions, thus the use of improved catalysts or an alternative approach is necessary (Tavasoli and Ozin 2018). During the reaction, carbon deposition on the surface of the catalyst is the most frequent reason for catalyst deactivation because the access of reactant molecules to the active metal sites was hampered (Li and Gong 2014). Thence, the solution to these issues is to develop catalysts and integrated reactor systems with high efficiency and specific selectivity to produce products with high conversion and minimal energy consumption among industrial time scales (Rodriguez et al., 2017; Liu et al., 2020a).
Among the various materials, the perovskites-type mixed oxides-based catalysts have become the focus of research due to their high-temperature thermochemical stability and high oxygen transport properties (Huang et al., 2018). Compared with traditionally supported catalysts, most of the active metals are substituted in the crystal structure and only a small fraction of active metals is on the surface in perovskites-type mixed oxides-based catalysts (Zhu et al., 2014; Wu et al., 2018). The substituted active metal particles would be exsolved to the surface under reduction atmosphere to gain highly dispersed metal crystals on the surface, which performed outstanding resistance to coarsening and agglomeration (Messaoudi et al., 2018). These inherent properties allow perovskite-type mixed oxides-based catalysts to have a wide range of applications in chemical catalysis (Ishikawa et al., 2020; Wang K et al., 2021; Zhu and Thomas 2009), electrochemical catalysis (Okamoto and Suzuki 2014; Yin et al., 2019), and photocatalysis (Peng et al., 2020; Xu R et al., 2020). As for the structural properties of perovskite-type mixed oxides-based catalysts, we will describe them in detail in the second section of this review.
CO2 hydrogenation and conversion technology need high temperatures to ensure thermodynamically favorable conditions, and naturally, lots of people have applied perovskite-type catalysts in this process (Su et al., 2016). Under the high reducing temperatures, the perovskite oxides are recognized to be partly reduced, leading to the formation of nanoparticles of B site metals, which are not only active for the reforming reaction but also insusceptible to carbon deposition (Jing et al., 2009; Tsiotsias et al., 2020). For example, le Saché et al. have applied a La2Zr2-xNixO7-δ pyrochlore-double perovskite catalyst for gas-phase CO2 recycling conversion, and the active Ni clusters were exsolved from pyrochlore-double perovskite materials after the reaction leading to highly dispersed active ensembles which account for the high activity and stability of the catalyst during CO2 recycling conversion reactions (le Saché et al., 2020). Valderrama et al. synthesized a series of perovskite-type oxides based on La-Sr-Co (La1-xSrxCoO3) used as precursors for the catalytic CO2 reforming of CH4, the Co0 nano-size particles are achieved and highly dispersed in the La2O2CO3-SrO solid matrix after activation/reduction process which leading to high activity performance (Valderrama et al., 2013). Perovskites-type based materials with a defined element have been reviewed for specific CO2 conversion reactions (Tabish et al., 2020; Madi et al., 2021), as far as we know, the review on the perovskites-type based catalysts for the thermal CO2 conversions has been rarely reported. . Here, we have especially attempted to expatiate on the catalytic pathways and the position of perovskite-type mixed oxides based catalysts in deciding the selectivity of CO2 hydrogenation and conversion to CO and CH4. In particular, we classify the main reactions for catalytic CO2 hydrogenation and conversion: 1) reverse water gas shift reaction (RWGS), 2) CO2 methanation, and 3) CO2 reforming of methane. We would provide an elaborated account of recent perovskite-type mixed oxides-based catalyst developments, together with the pathways and mechanisms of reactions. In addition to showing the latest optimal catalysts including their properties, we also provide the challenges that need to be dealt with and prospects for future research and development.
Perovskites-type mixed oxides-based catalysts
The performance of a catalyst largely depends on the structural and geometric parameters of the surface (Monteiro et al., 2019; Kopac et al., 2020; Riani et al., 2021). Apart from the traditionally supported catalysts, a class of crystalline oxide catalysts has attracted extensive attention due to their excellent thermal stability (Godding et al., 2019; Koch et al., 2020). In these materials, the active sites are incorporated into the structure, resulting in catalysts that are thermally stable at high temperatures. Moreover, a few of them possess instinctive oxygen mobility that can be strengthened by the replacement of active metals in the lattice, which is helpful to mitigate carbon deposition (Li M et al., 2020; Peng et al., 2020). Large numbers of these materials, such as perovskites (Huang et al., 2018; Ishikawa et al., 2020; Koch et al., 2020), pyrochlores (Li et al., 2016; Talanov and Talanov 2021; Trump et al., 2018; le Saché et al., 2018), fluorites (Chen et al., 2019; Gao et al., 2021), and hexa-aluminates (Tian et al., 2016; Xu L et al., 2020) have been investigated for varied high-temperature reactions.
Perovskite-type oxides (ABO3), which acquire the structure that large cation A locates on the edge and smaller cation B locating in the center of the octahedron, as shown in Figure 1A (Ji et al., 2020), are favorable materials to catalyze high-temperature reactions due to their tunable catalytic properties and thermal stabilities. Generally, the A site is filled with lanthanide metals (La, Nd, Sm, etc.) or alkaline earth metals (Sr, Ca, etc.), and the B site element is chosen from the transition metals (Fe, Ni, Mn, etc.) (Shin et al., 2016; Mateo et al., 2021). Another class of crystalline oxide materials with the general formula A2B2O7 has been used for CO2 conversion reactions (Kumar et al., 2016; Fang et al., 2021). The metals of the framework are similar to those of perovskite-type mixed oxides (ABO3) based materials and its model, as shown in Figure 1B. Namely, the larger rare-earth trivalent metal like La is at the A position, and the smaller tetravalent transition metal like Zr, Ti occupies the B site of these materials. However, the formation of the crystal phase depends on the ionic radius ratio of A-position to B-position: when the ratio is over 1.8, a perovskite structure appears; if the ratio is in the range of 1.4–1.8, pyrochlore is the dominant structure; and the fluorite phase prevails when the ratio is less than 1.4 (Pakhare and Spivey 2014).
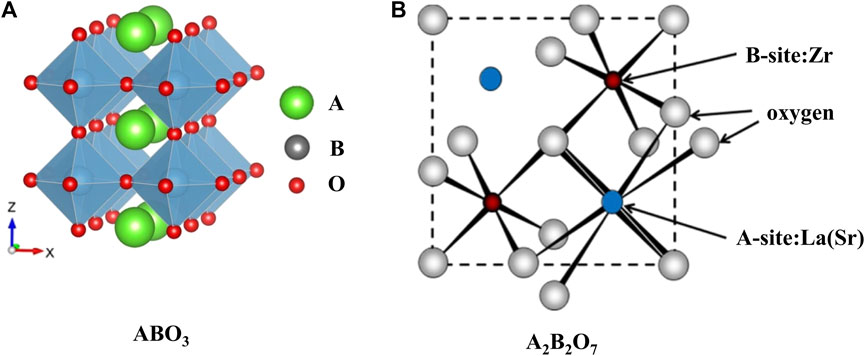
FIGURE 1. Ideal models of ABO3 (A) ((Ji et al., 2020); Copyright © 2020 (Royal Society of Chemistry) and A2B2O7 (B) [(Pakhare and Spivey 2014); Copyright © 2014 (Royal Society of Chemistry)] structure.
The crystalline oxides can be prepared using the Pechini sol-gel method (Haynes et al., 2008; Haynes et al., 2009; Blanco et al., 2022). Ethylene glycol or citric acid are used as complexing materials to mingle with the metal precursors (Li S et al., 2020; Onrubia-Calvo et al., 2021). The resulting amorphous resins, which are precursors of the perovskites, pyrochlores, or fluorites, are then calcined at high temperatures (usually 800–1000°C) to remove the remaining organics and construct the crystallographic ABO3 or A2B2O7 phase (Haynes et al., 2009; Kumar et al., 2016). The catalytic activity of ABO3 or A2B2O7 can be modulated by partial replacement of cations at the A and/or B sites, leading to the formation of structural defects to stabilize the uncommon oxidation states by B site components (Hare et al., 2018; Jiang et al., 2021; Zhang J et al., 2021). The appealing properties of crystalline oxides for catalytic reactions involve the high oxygen movability and stability of uncommon oxidation states in the structure, as well as high-temperature thermal stability (Su et al., 2014; Bai et al., 2019). In both pyrochlores and perovskites, most of the active metals are replaced inside the body of the crystal structure, except for a small percentage at the surface (Moradi et al., 2012; Bhavani et al., 2013; Valderrama et al., 2018). Under a reducing atmosphere, the transition metals could be exsolved to the surface of oxide to form highly dispersed crystals, meanwhile, the reduced states can be used as supported catalysts too (Valderrama et al., 2005; Valderrama et al., 2013; Yang et al., 2018). As the exsolution method can immobilize the particles more firmly on the support than the impregnation method, the exsolved particles have outstanding insusceptibility to coarsening and agglomeration. Therefore, the catalytic activity of the exsolved particles is more stable during the reaction operation. Moreover, the highly dispersed particles inhibit the formation of carbon deposition, thusly preventing the deactivation of catalysts (Jing et al., 2009; Wang X et al., 2019; Lim et al., 2021a).
Perovskites-type mixed oxides-based catalysts applied in CO2 conversion
We discuss the CO2 conversions, namely 1) reverse water gas shift reaction (RWGS), 2) CO2 methanation reaction, and 3) CO2 reforming of methane to form target products mainly over perovskites-type mixed oxides based catalysts. Before discussing the reaction performance of the crystalline oxide catalyst, we first briefly introduce the CO2 conversion reactions. Subsequently, we introduce the application of perovskite-type mixed oxides-based catalysts in CO2 conversion reactions, especially regarding the modification of perovskite with improving the reaction performance. Finally, we give an outlook on the future application of perovskite catalysts in CO2 conversions.
RWGS reaction
The hydrogenation of CO2 to CO, commonly referred to as the reverse water gas shift reaction (RWGS), is one of the most technically achievable reactions to realize the clean utilization of CO2 as an abundant renewable carbon source (Chen et al., 2020; Wang X et al., 2021). Apart from generating CO, this reaction may also be regarded as an intermediate process (e.g., CO2 methanation) for supplementary fuel and chemical synthesis (Hare et al., 2019a). The RWGS reaction is a reversible and energy-intensive way (Eq. 1), and its conversion of CO2 and selectivity of CO are typically determined by thermodynamic equilibrium (Liu et al., 2020b).
Owing to its endothermic property, the RWGS reaction is typically operated at high temperatures (up to 700 K) to achieve a satisfactory CO2 conversion (Liu et al., 2022). However, it could suffer the effect of catalyst sintering deactivation at elevated temperatures. Therefore, improving the catalytic activity at lower temperatures or adopting catalysts with higher temperature stability is the main strategy to realize the industrialization of the RWGS reaction (Kopac et al., 2020; Yang et al., 2020; Lim et al., 2021b; Jo et al., 2022). In any case, green hydrogen is needed for the RWGS when this process is envisaged as a greenhouse gas conversion route (Nityashree et al., 2020).
RWGS reaction on catalysts mainly proceeds through the redox mechanism or the formate dissociation progress (Chen et al., 2020). As shown in Figure 2, there are two main reaction pathways reported in literature 1) Formate pathway: goes on via more reactive carboxyl (*COOH) or formate (*HCOO) intermediates; 2) C-O bond cleavage pathway: CO2 is directly decomposed into *CO and *O. In the metal oxide systems, the metals adsorb dissociative H2 and spill it to the M-O sites in which CO2 is adsorbed (Lindenthal et al., 2020). Typically, in perovskite oxide (ABO3), CO2 conversion occurs on oxygen vacancies, and oxygen-deficient structures (ABO3-δ) are formed by reduced H2 (Kopac et al., 2020). According to all the catalysts reported so far, it is indicated that both mechanisms are common in any reaction, and which route has a relative advantage over the other depends on the specific catalyst (Thalinger et al., 2015).
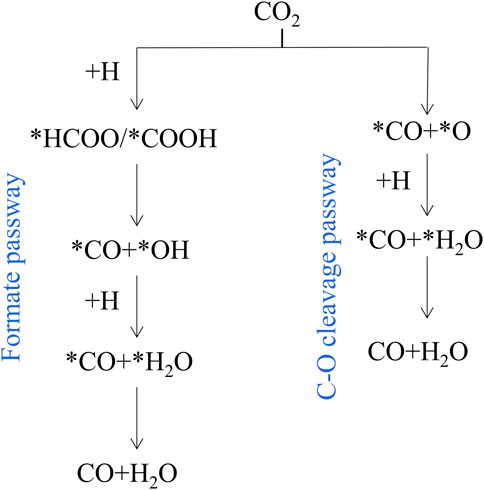
FIGURE 2. Simplified RWGS mechanism. Reproduced from Kattel et al. (2017); Copyright © 2017 (American Chemical Society).
Considering the importance of oxygen vacancies in the RWGS reaction, perovskite-like structure materials with high content of oxygen vacancies have been promising candidates (Maiti et al., 2018). Perovskite oxides (ABO3) are readily doped with highly reactive elements, these dopants escape from the perovskite lattice or form nanoparticles through diffusion (by exsolution) after controlled reduction or during the reaction, which leads to more oxygen vacancies generated thereby increasing the performance of RWGS reaction (Lindenthal et al., 2020). On the other hand, the exsolution of active metal well dispersed on perovskite surface, which is very beneficial to improve the catalytic activity, an example is shown in Figure 3 (Lindenthal et al., 2021). Analogously, Kuhn et al. synthesized five various Sr-doped lanthanum cobaltates, La1-XSrXCoO3−δ (0 ≤ X ≤ 1, with a step size of 0.25), to evaluate their carbon dioxide conversion properties. The result indicated that when X was 0.25, the La0.75Sr0.25CoO3−δ sample carried the best structure stability under reducing conditions and the top CO generation ability during the CO2 reoxidation process (Daza et al., 2014). Meanwhile, they also found the strontium-doped La0.75Sr0.25FeO3 (LSF) perovskite-type oxide combined with silica promoted a prominent extent of oxygen vacancies in the active phase, and concomitant with a decreased average LSF crystallite size, resulting in unprecedented rates of reverse water gas shift chemical looping. Furthermore, the support SiO2 could also suppress the perovskite sintering through the interfacial wettability effect which is confirmed by visual examination of microscopy. The inhibiting species of FeSiO3 and La2SiO5, which may lead to interfacial energy barriers and thereby limit accessibility to active surfaces, can restrict its formation by adjusting the mass ratio of perovskite and support silica (Hare et al., 2018). The combination of different supports and different morphologies of perovskites produces unexpected outcomes, which in turn exhibit different RWGS properties. For example, the researchers also developed the effect of using various supports (CeO2, Al2O3, SiO2, TiO2, and ZrO2) in combination with perovskite oxides for RWGS (Hare et al., 2018; Hare et al., 2019b). It is worth noting that the synthesis method of perovskite also influences the RWGS performance (Lim et al., 2021b; Jo et al., 2022).
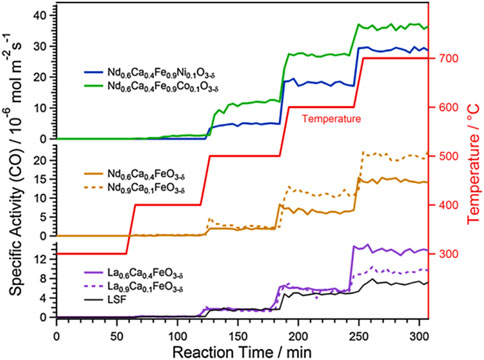
FIGURE 3. Comparison of RWGS reaction activity results on Co-, Ca-, and Ni-doped samples. Reproduced from Lindenthal et al. (2021); Copyright © 2021 (Elsevier).
The production of carbon monoxide via conventional, thermally driven RWGS is a costly process, requiring energy-intensive operating conditions. To decrease the operating temperature, Kawi et al. used non-thermal plasma (NTP) combine with perovskite La0.9Ce0.1B0.5B′0.5O3−δ-derived bimetallic catalysts (B: Cu, Ni, Fe, B’: Ni, Fe, Cu) formed a dielectric barrier discharge plasma-catalysis system to ignite RWGS reaction, the results revealed that the plasma-catalysis system has excellent capability to promote the RWGS reaction at low temperature and normal pressure (Liu et al., 2020b; Liu et al. 2020b; Liu et al. 2022). Furthermore, RWGS reaction with chemical looping (RWGS-CL) (Maiti et al., 2018; Lim et al., 2021b; Lee et al., 2022), which is comprised of a two-step redox step: reduction procedure by renewable H2 and oxidation step by CO2, would be a promising method because it can considerably reduce the operating temperature of the reduction process. High oxygen mobility of the perovskite oxides allows for the operation of these looping cycles without phase change of the oxides. The process is depicted in Figure 4A. The mechanism of RWGS-CL mainly relies on the generation of oxygen vacancies on these surfaces and the conversion of carbon dioxide to these oxygen vacancies. Therefore, probing these oxygen vacancies on different perovskite oxide compositions is essential to better formulate catalysts and understand their roles in CO2 conversion. Bhethanabotla et al. using density functional theory (DFT) calculated the oxygen vacancy formation energy in different perovskite oxides during CO2 conversion reaction (Figure 4B), and they found that using lanthanum and Ca-based perovskite oxides can achieve 100% selective CO generation at record low-temperature process temperatures of 450–500°C, and these materials performed very stably in several RWGS-CL cycles (Maiti et al., 2018).
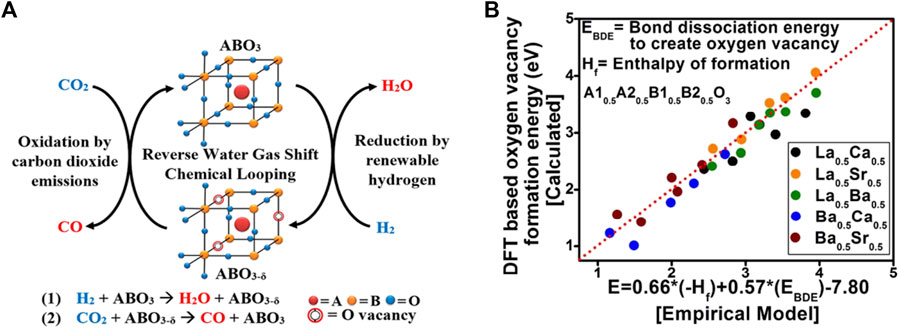
FIGURE 4. The process of RWGS-CL (A) Reproduced from (Hare et al., 2018); Copyright © 2018 (American Chemical Society) and the empirical modeling of oxygen vacancy formation energies (Evac) of the perovskite oxides (B) Reproduced from Maiti et al. (2018); Copyright ©2018 (Royal Society of Chemistry).
CO2 methanation to CH4
The CO2 methanation, also known as “Sabatier reaction”, was discovered by Sabatier et al., in 1902 (Senderens and Sabatier, 1902). From a thermodynamics perspective (Ye et al., 2019b), the enthalpy and Gibbs free energy of the CO2 methanation process are both negative, indicating a very favorable process (Eq. (2)) (Tsiotsias et al., 2020).
Although thermodynamically favored, the reaction is kinetically limited due to the high inertness of CO2. Indeed experimental CO2 methanation does not yield significant methane production at room temperature and atmospheric conditions (González-Castaño et al., 2021). Therefore, many studies have been carried out on CO2 methanation to CH4 in various types of catalytic systems (Wang et al., 2016; Rosid et al., 2019; Lv et al., 2020; Pastor-Pérez et al., 2020). However, CO2 methanation catalysts are prone to rapid and severe deactivation during the reaction process due to various physicochemical changes such as thermal degradation of support materials, metal sintering, and especially coke formation (le Saché et al., 2020; Sreedhar et al., 2019). Therefore, the development of effective and stable catalysts lefts a major challenge for CO2 methanation commercialization (Mebrahtu et al., 2018; Ashok et al., 2020).
In order to rationally design advanced catalytic systems, it is necessary to study the reaction mechanism of CO2 methanation (Lv et al., 2020). Roughly there are three potential reaction pathways well-accepted in literature: 1) RWGS pathway: Proceeds through *CO and then undergoes consecutive *CO hydrogenation via *HCO which ends up in *CHx species to produce methane. 2) C-O bond cleavage pathway: proceeds through direct dissociation of CO2 generates *CO and *O, and then *CO is further dissociated to *C and *O, the *C is hydrogenated to methane. 3) Formate pathway: proceeds through *HCOO and then consecutive hydrogenation via *H2CO and *H2COH which end up in *CH3 species to produce methane (Figure 5) (Aziz et al., 2015; Roy et al., 2018; Hussain et al., 2021). Typically, the presence of multiple active sites on the catalyst surface promotes the activation and dissociation of reactants to generate the desired product through different reaction intermediates (Rosid et al., 2019; Sreedhar et al., 2019; Riani et al., 2021).
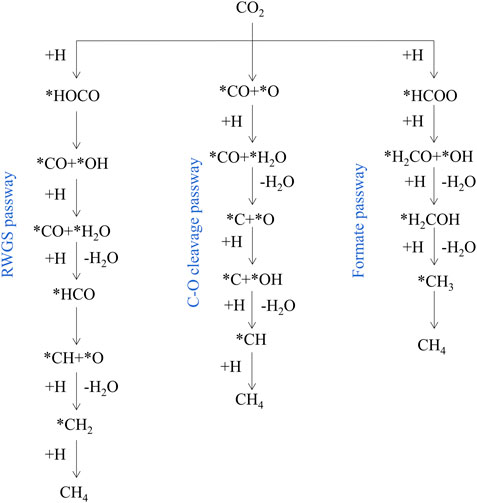
FIGURE 5. Simplified CO2 methanation reaction mechanism. Reproduced from Kattel et al. (2017); Copyright © 2017 (American Chemical Society).
The CO2 methanation reaction system is often accompanied by complex multiple side reactions, and the formation of coke from the side reactions is the main reason for the CO2 methanation catalysts' deactivation (Hussain et al., 2021). Another main CO2 methanation catalysts drawbacks concerning deactivation issues are high-temperature sintering (Roy et al., 2018). Therefore, the development of anti-carbon deposition and high-temperature sintering resistance catalysts is the key to solving the bottleneck of CO2 methanation (Ashok et al., 2020; Price et al., 2021). During the CO2 methanation reaction, oxygen vacancies usually play a role in favoring CO2 adsorption and enhancing the ability to resist carbon deposition (González-Castaño et al., 2021; Blanco et al., 2022). In addition, the oxygen vacancies are also considered to be the key factor for C-O dissociation obtaining higher CH4 yields (Wang et al., 2016). Considering the importance of oxygen vacancies, perovskite-like materials are promising candidates due to their the elevated oxygen mobility and high-temperature stability (González-Castaño et al., 2021). The generation, recovery, and regeneration of oxygen vacancies (cycle process) are often accompanied by the occurrence of redox reactions (Zhang J et al., 2021). It is found that the using (or doping) of variable valence metal in perovskite (A/B site) can accelerate the generation of oxygen vacancies or elevated oxygen mobility in the CO2 methanation (Li S et al., 2020; Zhang J et al., 2021). For example, M. González-Castaño et al. synthesized Ni catalyst supported on YMnO3 perovskite via coprecipitation method, and the replacement of Mn3+ by Ni2+ atoms result in the formation of Mn4+ species by way of a charge compensation mechanism, which attained the ability to exchange oxygen species, leading to the remarkable performance with TOFs = 20.1 s−1 at 400°C and 60 L/(g·h). The presence of oxygen vacancies in the YMnO3-x support effectively enhances the dissociative adsorption of CO2 through easier redox interconversion, resulting in high activity and stable catalytic behavior without evidence of deactivation (González-Castaño et al., 2021). Similarly, the variable valence metals like Ce (Ren et al., 2021; Zhang J et al., 2021), Fe (Thalinger et al., 2016b; Steiger et al., 2020), Ti (Tang et al., 2018; Do et al., 2020; Jiang et al., 2021), etc. can also lead to oxygen vacancies increase in perovskite structure materials.
It is found that the dispersion of active metals has a great influence on the performance of the CO2 methanation reaction (Lim et al., 2021a). In order to attain well-dispersed active metals, the metal loading on the catalyst is usually low to suppress agglomeration during the catalyst preparation. Low loading of active species would inevitably lead to relatively low activity; thus, the research focus is on fabrication catalysts with high dispersion under high loading (Jiang et al., 2021). In addition to oxygen mobility properties, perovskite oxides also exhibit good reactivity and thermal stability at higher metal loadings, so they are often used as redox catalysts in CO2 methanation reactions. Apart from increasing the dispersion of active metal by substitution (Lim et al., 2021a; Jiang et al., 2021), a variety of tandem catalysts consisting of two interfaces with a single structure has been recently designed and used to catalyze the continuous reaction of CO2 hydrogenation to methane (Do et al., 2020). Wang et al. utilize LaNiO3 with perovskite structure as a La-modified catalysts precursor to the synthesis of Ni-La2O3/SBA-15(C) for CO2 methanation. Owing to the LaNiO3 distinct perovskite structure, the interaction between La and Ni is enhanced, thereby reinforcing the synergistic effect of La2O3 and Ni, making Ni nanoparticles with high dispersibility as well as satisfactory resistance to sintering and carbon deposition. In addition, compared to the Ni-La2O3/SBA-15 catalyst synthesized by the traditional wet impregnation method, the Ni-La2O3/SBA-15(C) demonstrated a higher dispersion of Ni and displayed a better catalytic performance with a CO2 conversion of 90.7% and a CH4 selectivity of 99.5% at 320°C (Figure 6) (Wang X et al., 2019). Coincidentally, Liu G et al. (2020) also utilized the specific perovskite structure of LaNiO3/LaNi1-xCoxO3 to synthesize LaNi1-xCoxO3-based catalysts supported on mesostructured cellular foam (MCF) silica (LaNi1-xCoxO3/MCF) and evaluate its CO2 methanation performance. The highly dispersed La2O3 and Ni/Ni-co alloy nanoparticles were formed within the pores of the MCF support after reduction, which exhibited high performance (Zhang and Liu 2020a; Zhang and Liu 2020b). In addition to the above SBA-15 and MCF, using perovskite-structured LaNiO3 as the precursor, a high-performance CO2 methanation catalyst with highly dispersed Ni nanoparticles and strong metal interactions was prepared, which also appeared on the supports of CeO2 (Onrubia-Calvo et al., 2021), ZrO2 (Li S et al., 2020), SiO2 (Li S et al., 2019), γ-Al2O3 (Do et al., 2020) etc.
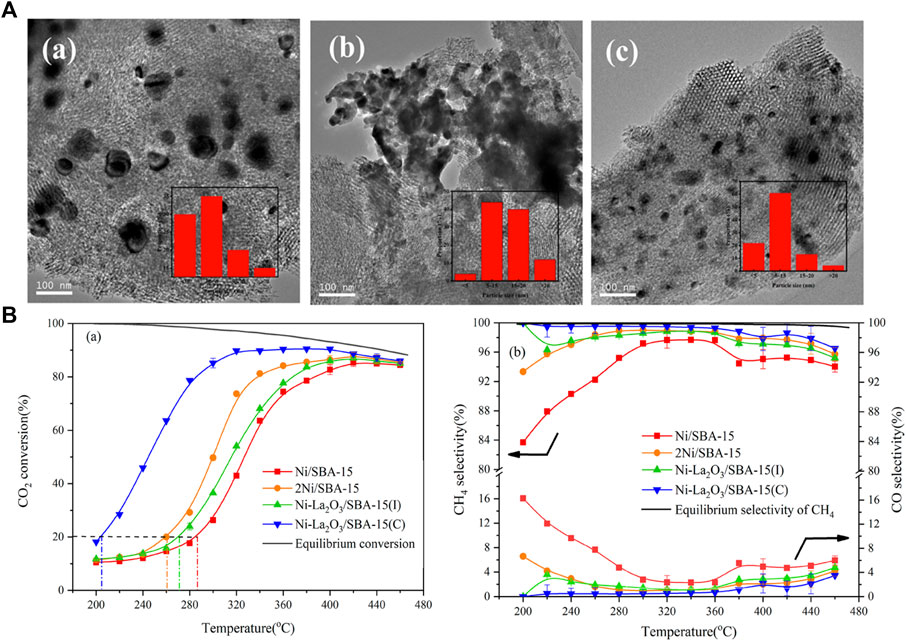
FIGURE 6. (A) TEM images for the catalysts after reaction: Ni/SBA-15 (a); Ni-La2O3/SBA-15(I) (b); Ni-La2O3/SBA-15(c). (B) Catalytic activity and selectivity of the catalysts. Reproduced from Wang X et al. (2019); Copyright © 2019 (American Chemical Society).
To avoid the agglomeration of active metal, it is desirable to have strong SMSI between the metal and support to achieve high performance (Shin et al., 2016; González-Castaño et al., 2021). Shin et al. study found that at the same loading of Co. and Pt (1 and 0.2 wt%, respectively), the barium zirconate support provides a more than six-fold increase in CH4 formation rate, accompanied by a high CH4 selectivity as compared to previously studied γ-Al2O3 supports at 325 °C. This enhancement is attributed to a strong interaction between the Co. particles and the BaZrO3 support, as well as atomically dispersing of the Pt. It is noted that Pt atoms decorating the surface Co/CoOx interface within the nanoparticle, and prefers to remain associated with the metallic Co. core as opposed to being incorporated into the CoOx shell during oxidation of the particle (Shin et al., 2016). However, Penner, et al. reported that strong metal-support interactions have limitations in complex metal-oxide systems. They employed Rh/Ni-La0.6Sr0.4FeO3-δ and Rh/Ni-SrTi0.7Fe0.3O3-δ as precursors to explore the relationship between metal-support interactions and the performance of CO2 methanation. The results exhibited there is no typical, reversible strong metal-support interaction during the reaction (Thalinger et al., 2016a; Thalinger et al., 2016b).
In addition to regular doping modification or using supports, novel strategies like special preparation methods or materials have emerged to improve the performance of CO2 methanation over perovskite structure (Arandiyan et al., 2018; Wang X et al., 2019). The colloidal crystal template method is widely used to create three-dimensional ordered macroporous (3DOM) structured materials due to its single-step low-temperature process, facile control in composition and morphology, and wide applicability to metal precursors. The interconnected porous network of 3DOM structured materials enables to control the active metal particle size and dispersion and influences the metal-support interaction during the exsolution process. For example, Rose Amal et.al using a poly(methyl methacrylate) microsphere colloidal crystal-templating route successfully prepared Ni-Rh nanoalloy/3DOM LaAlO3, the schematic illustration of in situ exsolution of the catalyst from an ABO3 perovskite structure shown in Figure 7A. The reduced Ni-Rh/3DOM LaAlO3 has high dispersion of bimetallic Ni-Rh NPs, rich surface adsorbed oxygen species and basic sites, and strong metal-support interaction after reduction treatment. The performance of CO2 conversion confirmed a significant enhancement in activity for the RhNi/3DOM LaAlO3 sample relative to the other catalysts (Figure 7B) (Arandiyan et al., 2018). Similarly, Wang et al. also used a template of poly methyl methacrylate colloidal crystal to synthesize Ni/Y2Zr2O7-3DOM, which has a much stronger interaction of NiO and the Y2Zr2O7-3DOM than Y2Zr2O7-CP support synthesized by a co-precipitation method. Under reducing conditions, the strong interaction of NiO and the Y2Zr2O7-3DOM achieved high active Ni surface and large quantities of surface-active O2−/alkaline sites, which are the mainly active agent trigger the CO2 methanation (Fang et al., 2021). Besides, the water is generated because of hydrogen oxidation and RWGS side reactions during CO2 methanation, which may cause the catalyst deactivation for not stable in water materials. For this, Kageyama et al. investigated the perovskite-type oxyhydride BaTiO2.4H0.6 as an effective water-stable support material for Ni-, Ru-based catalysts for CO2 methanation. The result proved that the oxyhydride support is 2–7 times more active for Ni and Ru than the oxide support of BaTiO3 (Tang et al., 2018).
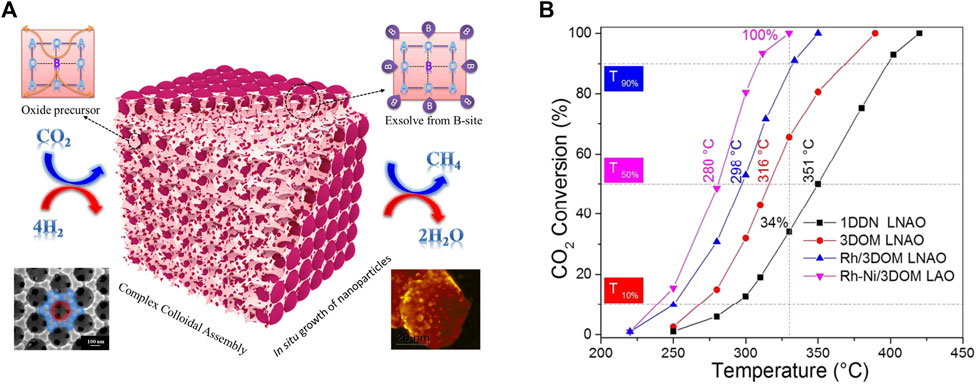
FIGURE 7. Schematic illustration of in situ exsolution of the catalyst from an ABO3 perovskite structure (A), and catalytic activities for the different catalysts (B). Reproduced from Arandiyan et al. (2018); Copyright © 2018 (American Chemical Society).
According to the mechanisms of CO2 methanation, it can be found that there is one mechanism through consecutive RWGS and CO hydrogenation. That means the selectivity of CO2 hydrogenation to CH4/CO can be adjusted by controlling the stability of the CO intermediate. It is found that crystalline oxide catalyst shows good catalytically active in both CO2 methanation and the RWGS routes and has different selectivity and activity under various reaction conditions (Tsiotsias et al., 2020). The products can be selectively controlled by adjusting the reaction temperature or the type of catalyst (Ma et al., 2019; Ho et al., 2020; Xu et al., 2022). For example, Chen et al. found that by changing the valence state of Ni, the product selectivity of CO2 hydrogenation can be adjusted on La-Fe-Ni perovskites, and the result suggests that higher-valence nickel-related species could produce more CO. They analyzed technology to illustrate that under reaction conditions, metallic Ni and higher-valence nickel-related species were formed on LaNiO3 and LaFe0.5Ni0.5O3, respectively. Furthermore, the DFT calculations indicate that CO is weakly bound to NiO (111), and the desorption of *CO is more favorable than its further hydrogenation to CH4, resulting in higher selectivity for CO (Figure 8) (Zhao et al., 2018). Similarly, Scott et al. have studied the product selectivity of CO2 hydrogenation by K cation substitution of La over LaNiO3 perovskite catalysts. It is found that when the potassium incorporation is up to 0.2, the La0.8K0.2NiO3 have the maximum amount of NiO in the catalyst which leads to an increase the CO selectivity. Therefore, keeping nickel-related species in higher-valence states under the reaction conditions is one of the important strategies in promoting CO selectivity (Tsounis et al., 2020).
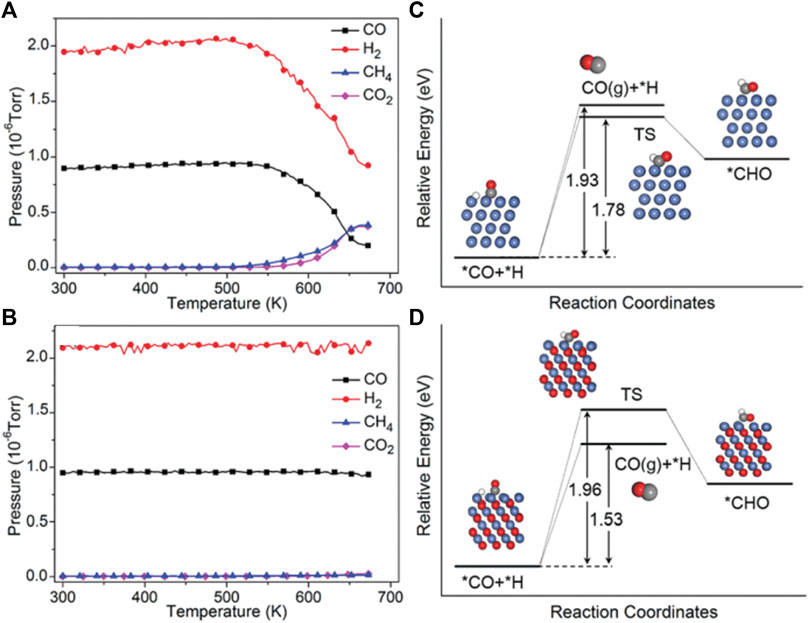
FIGURE 8. The partial pressures of CO, H2, CH4, and CO2 during the CO hydrogenation reaction for LaNiO3 (A) and LaFe0.5Ni0.5O3 (B). Potential energy diagram for the reaction routes of *CO + H on Ni (111) (C) and NiO (111) (D). Reproduced from Zhao et al. (2018); Copyright © 2018 (Royal Society of Chemistry).
CO2 reforming of CH4
The greenhouse gases of CH4 and CO2 are major contributors to global warming. The conversion of CH4 and CO2 to syngas (H2 + CO) has plentiful applications in synthetic chemistry (Li et al., 2021). Therefore, CO2 reforming of CH4 can not only alleviate global environmental problems but also provide a valuable chemical feedstock (Monteiro et al., 2019). It has been proved that the reserves of combustible ice (Gas Hydrate/Natural Gas Hydrate) in the South China Sea are as high as about 200 million cubic meters, equivalent to eight million tons of oil. Among many mining methods, the CO2 replacement method is a new mining method of combustible ice, which inevitably causes natural gas contaminated with CO2 in the product gas. Therefore, the efficient utilization of methane, especially the reforming of carbon dioxide, has attracted widespread attention. Simultaneously, the greenhouse gases (GHG) methane and carbon dioxide are the main “culprits” of global warming, their efficient use has always been a research focus (Wang et al., 2016; Wu et al., 2020).
CO2 reforming of CH4 is also called dry reforming of CH4 (DRM) due to not involving water in reactants, and it is an extremely endothermic reaction (Eq. (3)) (Abdullah et al., 2017). Therefore, it needs exceedingly high temperatures to achieve high equilibrium conversion of syngas at which supported metal catalysts are easily deactivated by sintering (le Saché and Reina 2022). Although the DRM produces H2/CO ratio with one theoretically, the simultaneous occurrence of side reactions of RWGS, CH4 decomposition (MD: Eq. 4), and the Boudouard reaction (BR: Eq. 5) causes the H2/CO ratio not close to one (Pakhare and Spivey 2014). Apart from affecting the ratio of synthesis gas, the occurrence of side reactions of the MD or BR can also lead to carbon deposition. Therefore, it is necessary to build up a thermally stable catalyst to suppress carbon deposition and sintering (Liu Y et al., 2020).
Typically, CH4 is activated on metals such as Rh, Pt, and Ni to produce carbon, CHx, or formyl intermediates, while CO2 is activated at the support or interface of the catalyst to form carbonate precursors (Wang et al., 2016; Li et al., 2021). During the DRM reaction, the reduction of CO2 to CO is accompanied by the generation of oxygen-containing species (or oxygen vacancies) and the enhancement of oxygen mobility, which is beneficial to the oxidation of surface carbon formed by CH4 activation, thereby eliminating carbon deposition (Monteiro et al., 2019). Based on this, the high oxygen mobility exhibited by perovskite-like materials makes them promissory candidates applied in DRM reactions (Bian et al., 2020; Bhattar et al., 2021). Besides, the high-temperature stability of perovskite-like materials further exacerbates their exploitation in DRM reactions (Shi et al., 2021). In general, LaNiO3 with perovskite’s structure is widely studied, which is usually decomposed to the Ni/La2O3 catalyst after H2 activation or DRM reaction. Over the LaNiO3 perovskites, the presumed mechanism is the adsorption of methane on metallic nickel particles and the subsequent cracking to form carbon deposits, which is recognized as the rate-determining step. At the same time, CO2 reacts with La2O3 to generate La2O2CO3 intermediate, which then reacts with carbon to form CO at the Ni0-La2O2CO3 interface accompanied by the recovery of Ni metal surface (Gallego et al., 2008; Moradi et al., 2010; Sadykov et al., 2013).
The use of hydrogen for pretreatment to obtain catalytically active metal oxide materials before DRM catalysis is still the preferred preparation method for promising perovskite-based DRM catalysts (Gallego et al., 2006). For example, the Rh substituted-La2Zr2O7 (pyrochlore-type) and La2Ti2O7 (perovskite-type) performed different DRM catalytic performances (Wu et al., 2018). Under reducing conditions, almost all Rh species substituted Zr made reactive oxygen species difficult to transfer, leading to the depositing of intermediate carbon on Rh-La2Zr2O7. On the contrast, part of Rh substituted Ti on Rh-La2Ti2O7 obtained coexistence of Rh0 and Rhδ+ after H2 was reduced, which accelerates the mobility of active O* and leading excellent activity and long-term stability for DRM (Figure 9). Therefore, the morphology and structural stability of perovskite-type mixed oxides-based materials also have a great impact on the DRM performance (Ho et al., 2020). Batiot-Dupeyrat et al. compared the La2NiO4 and LaNiO3 perovskite to be a precursor to exploring the performance of DRM. They found that after reduction treatment, La2NiO4 has the smallest nickel particles, making its catalytic activity higher than that of Ni/La2O3 or LaNiO3 (Gallego et al., 2006; Nezhad et al., 2021).
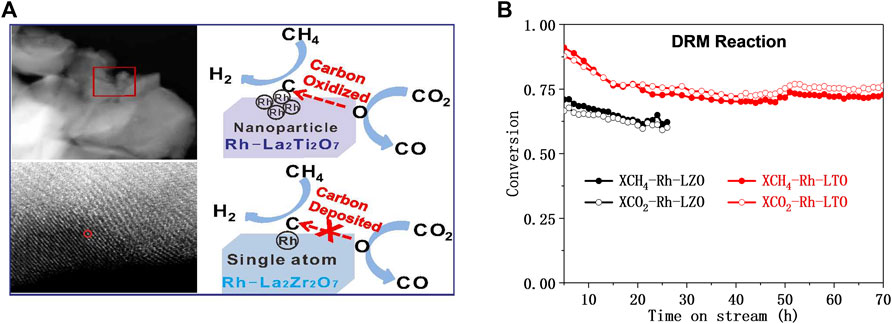
FIGURE 9. Schematic diagram of the DRM reaction (A) and the lifetime test on Rh substituted-La2B2O7 (B = Zr or Ti) (B). Reproduced from Wu et al. (2018); Copyright © 2018 (American Chemical Society).
In order to elevate the DRM activity and stability of perovskite structure materials, the most popular method is to do a part substitution. The most common substitution metals can be divided into alkaline earth metals (Mg, Ca, Sr, Ba et al.) (Dama et al., 2018; Bekheet et al., 2021), rare earth and variable valence metals (Sm, Ce, Nd, Gd, Cu, Mn, Cr et al.) (Moradi et al., 2012; Bhavani et al., 2013; Wang et al., 2018), VIII group (Fe, Co., Ni, Ru, Rh, Pd, Ir) (Wang H et al., 2019; Das et al., 2020; Managutti et al., 2021), and others (Moradi et al., 2014). The alkaline earth metals substitution perovskite always increases the basicity, the strong exsolved Ni particle/support interfacial interaction thereby the DRM catalytic activity (Yang et al., 2015; Wei et al., 2020). In the DRM redox reaction system, we usually replace a proper amount of site A/B with Ce (or Mn, Cu, etc. variable valence metal) in the mixed-oxide. Then the Ce3+/Ce4+ (or Mn2+/Mn3+/Mn4+, etc.) cations can reversibly shuttle between mixed-oxide and CeO2, which enhanced the oxygen vacancies or oxygen mobility and thereby improved the catalytic activity (Wang et al., 2018). The VIII group metals are usually an active site for CH4 decomposition. Thus, an appropriate substitution amount not only enhances the active metal exsolution but also improves the dispersion of active metal (Goldwasser et al., 2005; Oh et al., 2019). Furthermore, it is found that the use of silica materials (SBA-15, SiO2, etc.) (Rivas et al., 2010; Wang et al., 2013), SiC (Zhang Z et al., 2021), CeSiO2 (Rabelo-Neto et al., 2018), MgAl2O4 (Messaoudi et al., 2018), and Al2O3 (Moradi et al., 2013) as supports plays a role as promoters in the physicochemical and catalytic properties of the perovskite catalyst, especially the relatively high surface area of support promotes a highly dispersed and catalytic activity.
In addition to the commonly used Pechini sol-gel method to prepare perovskite structural materials, co-precipitation and impregnation method have also been widely studied, but their development is limited due to obtained smaller specific surface area of the catalyst and unsatisfactory activity on DRM (Rivas et al., 2008; Yadav and Das 2019; Yafarova et al., 2019). Therefore, new synthetic methods have emerged. For example, Joo et al. used atomic layer deposition (ALD)-combined topotactic exsolution method to obtain Ni-Fe alloy (Joo et al., 2020), in which raw materials are La0.6Sr0.2Ti0.85Ni0.15O3-δ and Fe2O3. The lower Ni-Fe alloy formation energy (-0.43 eV) enhanced the catalytic activity of DRM, prolonging its stability to 410 h (Joo et al., 2020; Ma et al., 2022). Figure 10 shows the process of conventional and topotactic exsolution via ALD. Other novel synthesis methods like the template method using SBA-15 as templating agent (Nair et al., 2014; Duan et al., 2017), ultrasonic spray pyrolysis method (Pereñíguez et al., 2010; Shahnazi and Firoozi 2021), microwave-assisted (Figueredo et al., 2018; Gangurde et al., 2018; Marin et al., 2021), magnetic distilled water-assisted (Mousavi and Nakhaei Pour, 2019; Mousavi et al., 2020), auto-combustion methods (Caprariis et al., 2015; Ruocco et al., 2019), one-step polymerization method (Silva et al., 2019) have also been performed in the preparation of efficient DRM catalysts. These newly developed synthesis methods could serve as a general powerhouse in other fields of energy utilization. In addition to improving the DRM activity from the perspective of catalysts, researchers have also tried to improve the performance of the reaction equipment and reaction conditions, such as using a plasma-assisted replaced heat source (Zheng et al., 2015), coupling chemical looping reforming, or autothermal reforming (Sastre et al., 2019).
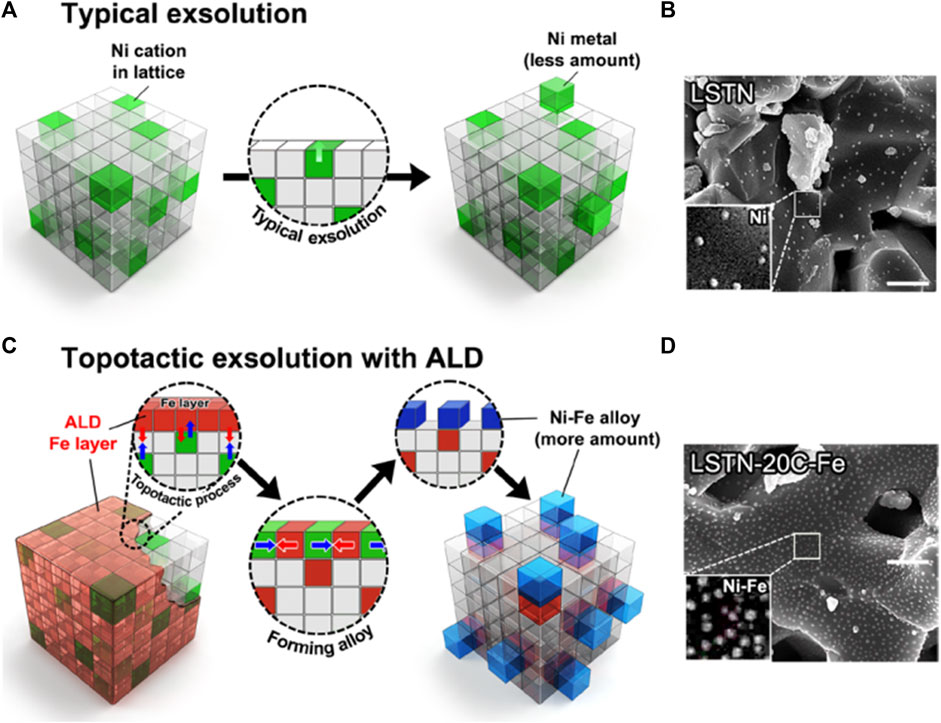
FIGURE 10. (A) Conventional exsolution for LSTN and (B) corresponding SEM image of LSTN. Scale bar, 500 nm. (C) Topotactic exsolution via ALD for LSTN-20C-Fe and (D) corresponding SEM image of LSTN-20C-Fe after reduction. Scale bar, 500 nm. Reproduced from Joo et al. (2020); Copyright © 2020 (Science).
Conclusions and perspectives
The increased amount of CO2 in the atmosphere mainly due to the excessive consumption of fossil fuels plays a major role in climate changes on a global scale. Therefore, it is mandatory to reduce CO2 emissions and develop CO2 capture as well as CO2 utilization technologies. The conversion and utilization of waste CO2 emissions into value-added products, such as chemicals, fuels, and other materials, while restraining climate change has drawn attention, which is crucial for a sustainable future.
Considering the high oxidation and thermodynamic stability of CO2, various strategies such as the catalyst preparation method, preparation conditions, and the component, as well the reaction conditions, technical approaches have been exploited in the conversion of CO2. In this review, we particularly elaborate on the perovskite-type mixed oxides-based catalysts on DRM, CO2 methanation, and RWGS reaction. All these gas-phase CO2 conversion processes are considered direct routes for CO2 valorization. The bottleneck for their implementation at the commercial scale is the lack of a robust and selective catalyst that can deliver the desired products satisfying the energy demands and favoring an economically viable chemical process. Herein perovskite catalysts emerge as fairly promising materials. given their defects chemistry with a significant concentration of oxygen vacancies and high-temperature stability characteristic of perovskite structure. Furthermore, the improved performance of the conversion of CO2 on perovskite-type mixed oxides-based catalysts by site A/B substitution, novel preparation method, combined with supports, etc., have been summarized. Apart from catalyst design, technical approaches involving innovative reactors and new processes design such as combined non-thermal plasma, light-drive, thermo-electric, etc., are also applied to improve CO2 conversion. Although it would take some time to bring these technologies up to the levels of practical CO2 hydrogenation, society’s need for effective measures is driving these rapid advances to reduce the acceleration in global warming caused by growing CO2 emissions.
For the future research in this field, we have proposed several perspectives as follows: 1) a more advanced preparation method should be developed for the perovskite-type mixed oxides based catalysts; 2) The relationship between the structure and catalytic performance over perovskite-type mixed oxides based catalysts for CO2 conversions should be investigated by the in-situ/operando characterization and DFT computational methods. The reaction mechanism of CO2 conversions is still challenging as the structure of perovskite-type mixed oxides based materials is complicated and the reaction pathway is diverse; 3) The combination of the perovskite-type mixed oxides based catalysts with other kinds of materials such as metal-organic frameworks, layered double hydroxide, and carbon materials could also be investigated to further enhance the catalytic performance for CO2 conversions; 4) Taking advantage of the optoelectronic properties of some perovskite-type mixed oxides based materials, future research could introduce solar-energy to drive catalysts for higher CO2 conversion efficiency; 5) Considering the remarkable oxygen mobility and redox cycle ability of perovskite-type mixed oxides based catalysts, the future reaction system could combine multiple technologies such as chemical looping or integrated reactor systems such as membrane reactors favoring one-step reaction and separation and leading to process intensification. All in all, these new technologies shall pursue the sustainable synthesis of added value products using CO2 as a carbon pool at high conversion with minimal energy consumption paving the way toward a net-zero modern society.
Author contributions
RY, XD, and ZF-Z contributed conception and design of the research; JW organized the database and wrote the draft of the manuscript; All authors contributed to the discussion and revision.
Funding
This research was funded by the Key Science and Technology Special Project of Anhui Province (Grant No. 202003b06020009), Scientific Research Team Project of Anhui Academy of Agricultural Sciences (Grant No. 2022YL020), and the National Natural Science Foundation of China (Grants No. 22005296).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, B., Abd Ghani, N. A., and Vo, D.-V. N. (2017). Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 162, 170–185. doi:10.1016/j.jclepro.2017.05.176
An, K., Zhang, S., Wang, J., Liu, Q., Zhang, Z., Liu, Y., et al. (2021). A highly selective catalyst of Co/La4Ga2O9 for CO2 hydrogenation to ethanol. J. Energy Chem. 56, 486–495. doi:10.1016/j.jechem.2020.08.045
Arandiyan, H., Wang, Y., Scott, J., Mesgari, S., Dai, H., Amal, R., et al. (2018). In situ exsolution of bimetallic Rh-Ni nanoalloys: A highly efficient catalyst for CO2 methanation. ACS Appl. Mat. Interfaces 10 (19), 16352–16357. doi:10.1021/acsami.8b00889
Ashok, J., Pati, S., Hongmanorom, P., Tianxi, Z., Junmei, C., Kawi, S., et al. (2020). A review of recent catalyst advances in CO2 methanation processes. Catal. Today 356, 471–489. doi:10.1016/j.cattod.2020.07.023
Aziz, M. A. A., Jalil, A. A., Triwahyono, S., and Ahmad, A. (2015). CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 17 (5), 2647–2663. doi:10.1039/c5gc00119f
Bai, Y., Wang, Y., Yuan, W., Sun, W., Zhang, G., Zheng, L., et al. (2019). Catalytic performance of perovskite-like oxide doped cerium (La2−xCexCoO4 ±y) as catalysts for dry reforming of methane. Chin. J. Chem. Eng. 27 (2), 379–385. doi:10.1016/j.cjche.2018.05.016
Bekheet, M. F., Delir Kheyrollahi Nezhad, P., Bonmassar, N., Schlicker, L., Gili, A., Praetz, S., et al. (2021). Steering the methane dry reforming reactivity of Ni/La2O3 catalysts by controlled in situ decomposition of doped La2NiO4 precursor structures. ACS Catal. 11 (1), 43–59. doi:10.1021/acscatal.0c04290
Bhattar, S., Abedin, M. A., Kanitkar, S., and Spivey, J. J. (2021). A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 365, 2–23. doi:10.1016/j.cattod.2020.10.041
Bhavani, A. G., Kim, W. Y., and Lee, J. S. (2013). Barium substituted lanthanum manganite perovskite for CO2 reforming of methane. ACS Catal. 3 (7), 1537–1544. doi:10.1021/cs400245m
Bian, Z., Wang, Z., Jiang, B., Hongmanorom, P., Zhong, W., Kawi, S., et al. (2020). A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 134, 110291. doi:10.1016/j.rser.2020.110291
Blanco, A., Caroca, J., Tamayo, R., Flores, M., Romero-Sáez, M., Espinoza-González, R., et al. (2022). CO2 methanation activity of Ni-doped perovskites. Fuel 320, 123954. doi:10.1016/j.fuel.2022.123954
Caprariis, B. d., Filippis, P. D., Petrullo, A., and Scarsella, M. (2015). Methane dry reforming over nickel perovsikite catalysts. Chem. Eng. Trans. 43, 991–996.
Chen, W., Chen, Y., Bu, X., Long, T., Zhang, G., Chen, F., et al. (2019). Rheological investigations on the hetero-coagulation between the fine fluorite and quartz under fluorite flotation-related conditions. Powder Technol. 354, 423–431. doi:10.1016/j.powtec.2019.06.019
Chen, X., Chen, Y., Song, C., Ji, P., Wang, N., Wang, W., et al. (2020). Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction. Front. Chem. 8, 709. doi:10.3389/fchem.2020.00709
Dama, S., Ghodke, S. R., Bobade, R., Gurav, H. R., and Chilukuri, S. (2018). Active and durable alkaline Earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B Environ. 224, 146–158. doi:10.1016/j.apcatb.2017.10.048
Das, S., Bhattar, S., Liu, L., Wang, Z., Xi, S., Spivey, J. J., et al. (2020). Effect of partial Fe substitution in La0.9Sr0.1NiO3 perovskite-derived catalysts on the reaction mechanism of methane dry reforming. ACS Catal. 10 (21), 12466–12486. doi:10.1021/acscatal.0c01229
Daza, Y. A., Kent, R. A., Yung, M. M., and Kuhn, J. N. (2014). Carbon dioxide conversion by reverse water–gas shift chemical looping on perovskite-type oxides. Ind. Eng. Chem. Res. 53 (14), 5828–5837. doi:10.1021/ie5002185
Do, J. Y., Park, N.-K., Seo, M. W., Lee, D., Ryu, H.-J., Kang, M., et al. (2020). Effective thermocatalytic carbon dioxide methanation on Ca-inserted NiTiO3 perovskite. Fuel 271, 117624. doi:10.1016/j.fuel.2020.117624
Duan, Q., Wang, J., Ding, C., Ding, H., Guo, S., Jia, Y., et al. (2017). Partial oxidation of methane over Ni based catalyst derived from order mesoporous LaNiO3 perovskite prepared by modified nanocasting method. Fuel 193, 112–118. doi:10.1016/j.fuel.2016.12.033
Fang, X., Xia, L., Li, S., Hong, Z., Yang, M., Xu, X., et al. (2021). Superior 3DOM Y2Zr2O7 supports for Ni to fabricate highly active and selective catalysts for CO2 methanation. Fuel 293, 120460. doi:10.1016/j.fuel.2021.120460
Figueredo, G. P., Medeiros, R. L. B. A., Macedo, H. P., de Oliveira, Â. A. S., Braga, R. M., Mercury, J. M. R., et al. (2018). A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3. Int. J. Hydrogen Energy 43 (24), 11022–11037. doi:10.1016/j.ijhydene.2018.04.224
Gallego, G. n. S., Batiot-Dupeyrat, C., Barrault, J. l., and Mondrago´n, F. (2008). Dual active-site mechanism for dry methane reforming over Ni/La2O3 produced from LaNiO3 perovskite. Ind. Eng. Chem. Res. 47, 9272–9278. doi:10.1021/ie800281t
Gallego, G. S., Mondragón, F., Barrault, J., Tatibouët, J.-M., and Batiot-Dupeyrat, C. (2006). CO2 reforming of CH4 over La–Ni based perovskite precursors. Appl. Catal. A General 311, 164–171. doi:10.1016/j.apcata.2006.06.024
Gangurde, L. S., Sturm, G. S. J., Valero-Romero, M. J., Mallada, R., Santamaria, J., Stankiewicz, A. I., et al. (2018). Synthesis, characterization, and application of ruthenium-doped SrTiO3 perovskite catalysts for microwave-assisted methane dry reforming. Chem. Eng. Process. - Process Intensif. 127, 178–190. doi:10.1016/j.cep.2018.03.024
Gao, S., Liu, N., Liu, J., Chen, W., Liang, X., Yuan, Y., et al. (2020). Synthesis of higher alcohols by CO hydrogenation over catalysts derived from LaCo1-xMnxO3 perovskites: Effect of the partial substitution of Co by Mn. Fuel 261, 116415. doi:10.1016/j.fuel.2019.116415
Gao, Z., Wang, C., Sun, W., Gao, Y., and Kowalczuk, P. B. (2021). Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 290, 102382. doi:10.1016/j.cis.2021.102382
Godding, J. S. W., Ramadan, A. J., Lin, Y.-H., Schutt, K., Snaith, H. J., Wenger, B., et al. (2019). Oxidative passivation of metal halide perovskites. Joule 3 (11), 2716–2731. doi:10.1016/j.joule.2019.08.006
Goldwasser, M. R., Rivas, M. E., Lugo, M. L., Pietri, E., Pérez-Zurita, J., Cubeiro, M. L., et al. (2005). Combined methane reforming in presence of CO2 and O2 over LaFe1−xCoxO3 mixed-oxide perovskites as catalysts precursors. Catal. Today 107-108, 106–113. doi:10.1016/j.cattod.2005.07.073
González-Castaño, M., de Miguel, J. C. N., Penkova, A., Centeno, M. A., Odriozola, J. A., Arellano-Garcia, H., et al. (2021). Ni/YMnO3 perovskite catalyst for CO2 methanation. Appl. Mater. Today 23, 101055. doi:10.1016/j.apmt.2021.101055
Hare, B. J., Maiti, D., Daza, Y. A., Bhethanabotla, V. R., and Kuhn, J. N. (2018). Enhanced CO2 conversion to CO by silica-supported perovskite oxides at low temperatures. ACS Catal. 8 (4), 3021–3029. doi:10.1021/acscatal.7b03941
Hare, B. J., Maiti, D., Meier, A. J., Bhethanabotla, V. R., and Kuhn, J. N. (2019a). CO2 conversion performance of perovskite oxides designed with abundant metals. Ind. Eng. Chem. Res. 58 (28), 12551–12560. doi:10.1021/acs.iecr.9b01153
Hare, B. J., Maiti, D., Ramani, S., Ramos, A. E., Bhethanabotla, V. R., Kuhn, J. N., et al. (2019b). Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides. Catal. Today 323, 225–232. doi:10.1016/j.cattod.2018.06.002
Haynes, D., Berry, D., Shekhawat, D., and Spivey, J. (2008). Catalytic partial oxidation of n-tetradecane using pyrochlores: Effect of Rh and Sr substitution. Catal. Today 136 (3-4), 206–213. doi:10.1016/j.cattod.2008.02.012
Haynes, D. J., Berry, D. A., Shekhawat, D., and Spivey, J. J. (2009). Catalytic partial oxidation of n-tetradecane using Rh and Sr substituted pyrochlores: Effects of sulfur. Catal. Today 145 (1-2), 121–126. doi:10.1016/j.cattod.2008.05.014
Ho, P. H., de Luna, G. S., Angelucci, S., Canciani, A., Jones, W., Decarolis, D., et al. (2020). Understanding structure-activity relationships in highly active La promoted Ni catalysts for CO2 methanation. Appl. Catal. B Environ. 278, 119256. doi:10.1016/j.apcatb.2020.119256
Hu, F., Ye, R., Lu, Z.-H., Zhang, R., and Feng, G. (2021). Structure–activity relationship of Ni-based catalysts toward CO2 methanation: Recent advances and future perspectives. Energy fuels. 36 (1), 156–169. doi:10.1021/acs.energyfuels.1c03645
Huang, X., Zhao, G., Wang, G., and Irvine, J. T. S. (2018). Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 9 (15), 3623–3637. doi:10.1039/c7sc03920d
Hussain, I., Jalil, A. A., Hassan, N. S., and Hamid, M. Y. S. (2021). Recent advances in catalytic systems for CO2 conversion to substitute natural gas (SNG): Perspective and challenges. J. Energy Chem. 62, 377–407. doi:10.1016/j.jechem.2021.03.040
Ishikawa, S., Noda, N., Wada, M., Tsurumi, S., and Ueda, W. (2020). Selective oxidation of methacrolein over crystalline Mo3VOx catalysts and comparison of their catalytic properties with heteropoly acid catalysts. ACS Catal. 10 (18), 10535–10545. doi:10.1021/acscatal.0c02530
Ji, Q., Bi, L., Zhang, J., Cao, H., and Zhao, X. S. (2020). The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 13 (5), 1408–1428. doi:10.1039/d0ee00092b
Jiang, K., Men, Y., Liu, S., Wang, J., An, W., Yu, H., et al. (2021). Highly stable and selective CoxNiyTiO3 for CO2 methanation: Electron transfer and interface interaction. J. CO2 Util. 53, 101743. doi:10.1016/j.jcou.2021.101743
Jing, G., Li-shan, J., Wei-ping, F., Qing-biao, L., and Hao, S. (2009). Methanation of carbon dioxide over the LaNiO3 perovskite catalysts activated under the reactant stream. J. Fuel Chem. Technol. 37 (5), 573–577. doi:10.1016/s1872-5813(10)60008-4
Jo, A., Kim, Y., Lim, H. S., Lee, M., Kang, D., Lee, J. W., et al. (2022). Controlled template removal from nanocast La0.8Sr0.2FeO3 for enhanced CO2 conversion by reverse water gas shift chemical looping. J. CO2 Util. 56, 101845. doi:10.1016/j.jcou.2021.101845
Joo, S., Seong, A., Kwon, O., Kim, K., Lee, J. H., Gorte, R. J., et al. (2020). Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 6, eabb1573. doi:10.1126/sciadv.abb1573
Kattel, S., Liu, P., and Chen, J. G. (2017). Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 139 (29), 9739–9754. doi:10.1021/jacs.7b05362
Koch, G., Hävecker, M., Teschner, D., Carey, S. J., Wang, Y., Kube, P., et al. (2020). Surface conditions that constrain alkane oxidation on perovskites. ACS Catal. 10 (13), 7007–7020. doi:10.1021/acscatal.0c01289
Kopac, D., Likozar, B., and Hus, M. (2020). How size matters: Electronic, cooperative, and geometric effect in perovskite-supported copper catalysts for CO2 reduction. ACS Catal. 10 (7), 4092–4102. doi:10.1021/acscatal.9b05303
Kumar, N., Roy, A., Wang, Z., L’Abbate, E. M., Haynes, D., Shekhawat, D., et al. (2016). Bi-reforming of methane on Ni-based pyrochlore catalyst. Appl. Catal. A General 517, 211–216. doi:10.1016/j.apcata.2016.03.016
le Saché, E., Pastor-Pérez, L., Haycock, B. J., Villora-Picó, J. J., Sepúlveda-Escribano, A., Reina, T. R., et al. (2020). Switchable catalysts for chemical CO2 recycling: A step forward in the methanation and reverse water–gas shift reactions. ACS Sustain. Chem. Eng. 8 (11), 4614–4622. doi:10.1021/acssuschemeng.0c00551
le Saché, E., Pastor-Pérez, L., Watson, D., Sepúlveda-Escribano, A., and Reina, T. R. (2018). Ni stabilised on inorganic complex structures: Superior catalysts for chemical CO2 recycling via dry reforming of methane. Appl. Catal. B Environ. 236, 458–465. doi:10.1016/j.apcatb.2018.05.051
le Saché, E., and Reina, T. R. (2022). Analysis of Dry Reforming as direct route for gas phase CO2 conversion. The past, the present and future of catalytic DRM technologies. Prog. Energy Combust. Sci. 89, 100970. doi:10.1016/j.pecs.2021.100970
Lee, M., Kim, Y., Lim, H. S., Jo, A., Kang, D., Lee, J. W., et al. (2022). Low temperature CO2 conversion facilitated by the preserved morphology of metal oxide-perovskite composite. Chem. Eng. J. 437, 135151. doi:10.1016/j.cej.2022.135151
Li, F., Dong, X., Zhao, N., and Xiao, F. (2019). Influence of NaBH4 liquid reduction over LaCuZn perovskite for CO2 hydrogenation to methanol. Catal. Lett. 150 (4), 922–929. doi:10.1007/s10562-019-03032-x
Li, F. Y., Li, Y. D., Kim, Y. B., Balents, L., Yu, Y., Chen, G., et al. (2016). Weyl magnons in breathing pyrochlore antiferromagnets. Nat. Commun. 7, 12691. doi:10.1038/ncomms12691
Li, F., Zhan, H., Zhao, N., and Xiao, F. (2017). CO2 hydrogenation to methanol over La-Mn-Cu-Zn-O based catalysts derived from perovskite precursors. Int. J. Hydrogen Energy 42 (32), 20649–20657. doi:10.1016/j.ijhydene.2017.06.200
Li, M., Niu, H., Druce, J., Tellez, H., Ishihara, T., Kilner, J. A., et al. (2020). A CO2-tolerant perovskite oxide with high oxide ion and electronic conductivity. Adv. Mat. 32 (4), e1905200. doi:10.1002/adma.201905200
Li, M., Sun, Z., and Hu, Y. H. (2021). Catalysts for CO2 reforming of CH4: A review. J. Mat. Chem. A Mat. 9 (21), 12495–12520. doi:10.1039/d1ta00440a
Li, Q. (2021). The view of technological innovation in coal industry under the vision of carbon neutralization. Int. J. Coal Sci. Technol. 8 (6), 1197–1207. doi:10.1007/s40789-021-00458-w
Li, S., and Gong, J. (2014). Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 43 (21), 7245–7256. doi:10.1039/c4cs00223g
Li, S., Guo, S., Gong, D., Kang, N., Fang, K.-G., Liu, Y., et al. (2019). Nano composite composed of MoOx-La2O3 Ni on SiO2 for storing hydrogen into CH4 via CO2 methanation. Int. J. Hydrogen Energy 44 (3), 1597–1609. doi:10.1016/j.ijhydene.2018.11.130
Li, S., Liu, G., Zhang, S., An, K., Ma, Z., Wang, L., et al. (2020). Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 43, 155–164. doi:10.1016/j.jechem.2019.08.024
Lim, H. S., Kim, G., Kim, Y., Lee, M., Kang, D., Lee, H., et al. (2021a). Ni-exsolved La1-xCaxNiO3 perovskites for improving CO2 methanation. Chem. Eng. J. 412, 127557. doi:10.1016/j.cej.2020.127557
Lim, H. S., Kim, Y., Kang, D., Lee, M., Jo, A., Lee, J. W., et al. (2021b). Fundamental aspects of enhancing low-temperature CO2 splitting to CO on a double La2NiFeO6 perovskite. ACS Catal. 11 (19), 12220–12231. doi:10.1021/acscatal.1c03398
Lindenthal, L., Popovic, J., Rameshan, R., Huber, J., Schrenk, F., Ruh, T., et al. (2021). Novel perovskite catalysts for CO2 utilization-Exsolution enhanced reverse water-gas shift activity. Appl. Catal. B Environ. 292, 120183. doi:10.1016/j.apcatb.2021.120183
Lindenthal, L., Rameshan, R., Summerer, H., Ruh, T., Popovic, J., Nenning, A., et al. (2020). Modifying the surface structure of perovskite-based catalysts by nanoparticle exsolution. Catalysts 10 (3), 268. doi:10.3390/catal10030268
Liu, G., Ariyarathna, I. R., Ciborowski, S. M., Zhu, Z., Miliordos, E., Bowen, K. H., et al. (2020). Simultaneous functionalization of methane and carbon dioxide mediated by single platinum atomic anions. J. Am. Chem. Soc. 142 (51), 21556–21561. doi:10.1021/jacs.0c11112
Liu, L., Dai, J., Yang, Z., Li, Y., Su, X., Zhang, Z., et al. (2022). Plasma-catalytic carbon dioxide conversion by reverse water–gas shift over La0.9Ce0.1B0.5B’0.5O3-δ perovskite-derived bimetallic catalysts. Chem. Eng. J. 431, 134009. doi:10.1016/j.cej.2021.134009
Liu, L., Das, S., Chen, T., Dewangan, N., Ashok, J., Xi, S., et al. (2020a). Low temperature catalytic reverse water-gas shift reaction over perovskite catalysts in DBD plasma. Appl. Catal. B Environ. 265, 118573. doi:10.1016/j.apcatb.2019.118573
Liu, L., Zhang, Z., Das, S., Xi, S., and Kawi, S. (2020b). LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Convers. Manag. 206, 112475. doi:10.1016/j.enconman.2020.112475
Liu, Y., Deng, D., and Bao, X. (2020). Catalysis for selected C1 chemistry. Chem 6 (10), 2497–2514. doi:10.1016/j.chempr.2020.08.026
Lv, C., Xu, L., Chen, M., Cui, Y., Wen, X., Li, Y., et al. (2020). Recent progresses in constructing the highly efficient Ni based catalysts with advanced low-temperature activity toward CO2 methanation. Front. Chem. 8, 269. doi:10.3389/fchem.2020.00269
Ma, Y., Liu, J., Chu, M., Yue, J., Cui, Y., Xu, G., et al. (2019). Cooperation between active metal and basic support in Ni-based catalyst for low-temperature CO2 methanation. Catal. Lett. 150 (5), 1418–1426. doi:10.1007/s10562-019-03033-w
Ma, Y., Su, P., Ge, Y., Wang, F., Xue, R., Wang, Z., et al. (2022). A novel LaAlO3 perovskite with large surface area supported Ni-based catalyst for methane dry reforming. Catal. Lett., 1–11. doi:10.1007/s10562-021-03910-3
Madi, M., Tahir, M., and Tasleem, S. (2021). Advances in structural modification of perovskite semiconductors for visible light assisted photocatalytic CO2 reduction to renewable solar fuels: A review. J. Environ. Chem. Eng. 9 (5), 106264. doi:10.1016/j.jece.2021.106264
Maiti, D., Hare, B. J., Daza, Y. A., Ramos, A. E., Kuhn, J. N., Bhethanabotla, V. R., et al. (2018). Earth abundant perovskite oxides for low temperature CO2 conversion. Energy Environ. Sci. 11 (3), 648–659. doi:10.1039/c7ee03383d
Managutti, P. B., Tymen, S., Liu, X., Hernandez, O., Prestipino, C., Salle, A. L. G. L., et al. (2021). Exsolution of Ni nanoparticles from A-site-deficient layered double perovskites for dry reforming of methane and as an anode material for a solid oxide fuel cell. ACS Appl. Mat. Interfaces 13 (30), 35719–35728. doi:10.1021/acsami.1c08158
Marin, C. M., Popczun, E. J., Nguyen-Phan, T.-D., Tafen, D. N., Alfonso, D., Waluyo, I., et al. (2021). Designing perovskite catalysts for controlled active-site exsolution in the microwave dry reforming of methane. Appl. Catal. B Environ. 284, 119711. doi:10.1016/j.apcatb.2020.119711
Mateo, D., Maity, P., Shterk, G., Mohammed, O. F., and Gascon, J. (2021). Tunable selectivity in CO2 photo-thermal reduction by perovskite-supported Pd nanoparticles. ChemSusChem 14 (24), 5525–5533. doi:10.1002/cssc.202101950
Mebrahtu, C., Abate, S., Perathoner, S., Chen, S., and Centi, G. (2018). CO2 methanation over Ni catalysts based on ternary and quaternary mixed oxide: A comparison and analysis of the structure-activity relationships. Catal. Today 304, 181–189. doi:10.1016/j.cattod.2017.08.060
Messaoudi, H., Thomas, S., Djaidja, A., Slyemi, S., and Barama, A. (2018). Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 24, 40–49. doi:10.1016/j.jcou.2017.12.002
Monteiro, W. F., Vieira, M. O., Calgaro, C. O., Perez-Lopez, O. W., and Ligabue, R. A. (2019). Dry reforming of methane using modified sodium and protonated titanate nanotube catalysts. Fuel 253, 713–721. doi:10.1016/j.fuel.2019.05.019
Moradi, G., Hemmati, H., and Rahmanzadeh, M. (2013). Preparation of a LaNiO3/γ-Al2O3 catalyst and its performance in dry reforming of methane. Chem. Eng. Technol. 36 (4), 575–580. doi:10.1002/ceat.201200555
Moradi, G. R., Khosravian, F., and Rahmanzadeh, M. (2012). Effects of partial substitution of Ni by Cu in LaNiO3 perovskite catalyst for dry methane reforming. Chin. J. Catal. 33 (4-6), 797–801. doi:10.1016/s1872-2067(11)60378-1
Moradi, G. R., Rahmanzadeh, M., and Khosravian, F. (2014). The effects of partial substitution of Ni by Zn in LaNiO3 perovskite catalyst for methane dry reforming. J. CO2 Util. 6, 7–11. doi:10.1016/j.jcou.2014.02.001
Moradi, G. R., Rahmanzadeh, M., and Sharifnia, S. (2010). Kinetic investigation of CO2 reforming of CH4 over La–Ni based perovskite. Chem. Eng. J. 162 (2), 787–791. doi:10.1016/j.cej.2010.06.006
Mousavi, M., Nakhaei Pour, A., Gholizadeh, M., Mohammadi, A., and Kamali Shahri, S. M. (2020). Dry reforming of methane by La0.5Sr0.5NiO3 perovskite oxides: Influence of preparation method on performance and structural features of the catalysts. J. Chem. Technol. Biotechnol. 95 (11), 2911–2920. doi:10.1002/jctb.6451
Mousavi, M., and Nakhaei Pour, A. (2019). Performance and structural features of LaNi0.5Co0.5O3 perovskite oxides for the dry reforming of methane: Influence of the preparation method. New J. Chem. 43 (27), 10763–10773. doi:10.1039/c9nj01805k
Nair, M. M., Kaliaguine, S., and Kleitz, F. (2014). Nanocast LaNiO3 perovskites as precursors for the preparation of coke-resistant dry reforming catalysts. ACS Catal. 4 (11), 3837–3846. doi:10.1021/cs500918c
Nezhad, P. D. K., Bekheet, M. F., Bonmassar, N., Schlicker, L., Gili, A., Kamutzki, F., et al. (2021). Mechanistic in situ insights into the formation, structural and catalytic aspects of the La2NiO4 intermediate phase in the dry reforming of methane over Ni-based perovskite catalysts. Appl. Catal. A General 612, 117984. doi:10.1016/j.apcata.2020.117984
Nityashree, N., Price, C. A. H., Pastor-Perez, L., Manohara, G. V., Garcia, S., Maroto-Valer, M. M., et al. (2020). Carbon stabilised saponite supported transition metal-alloy catalysts for chemical CO2 utilisation via reverse water-gas shift reaction. Appl. Catal. B Environ. 261, 118241. doi:10.1016/j.apcatb.2019.118241
Oh, J. H., Kwon, B. W., Cho, J., Lee, C. H., Kim, M. K., Choi, S. H., et al. (2019). Importance of exsolution in transition-metal (Co, Rh, and Ir)-Doped LaCrO3 perovskite catalysts for boosting dry reforming of CH4 using CO2 for hydrogen production. Ind. Eng. Chem. Res. 58 (16), 6385–6393. doi:10.1021/acs.iecr.8b05337
Okamoto, Y., and Suzuki, Y. (2014). Perovskite-type SrTiO3, CaTiO3 and BaTiO3 porous film electrodes for dye-sensitized solar cells. J. Ceram. Soc. Japan 122 (1428), 728–731.
Onrubia-Calvo, J. A., Pereda-Ayo, B., González-Marcos, J. A., Bueno-López, A., and González-Velasco, J. R. (2021). Design of CeO2-supported LaNiO3 perovskites as precursors of highly active catalysts for CO2 methanation. Catal. Sci. Technol. 11 (18), 6065–6079. doi:10.1039/d1cy00659b
Pakhare, D., and Spivey, J. (2014). A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43 (22), 7813–7837. doi:10.1039/c3cs60395d
Pastor-Pérez, L., Patel, V., Le Saché, E., and Reina, T. R. (2020). CO2 methanation in the presence of methane: Catalysts design and effect of methane concentration in the reaction mixture. J. Energy Inst. 93 (1), 415–424. doi:10.1016/j.joei.2019.01.015
Peng, B., Hu, Y., Murakami, S., Zhang, T., and Monserrat, B. (2020). Topological phonons in oxide perovskites controlled by light. Sci. Adv. 6, eabd1618. doi:10.1126/sciadv.abd1618
Pereñíguez, R., González-DelaCruz, V. M., Holgado, J. P., and Caballero, A. (2010). Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B Environ. 93 (3-4), 346–353. doi:10.1016/j.apcatb.2009.09.040
Price, C. A. H., Reina, T. R., and Liu, J. (2021). Engineering heterogenous catalysts for chemical CO2 utilization: Lessons from thermal catalysis and advantages of yolk@shell structured nanoreactors. J. Energy Chem. 57, 304–324. doi:10.1016/j.jechem.2020.08.061
Rabelo-Neto, R. C., Sales, H. B. E., Inocêncio, C. V. M., Varga, E., Oszko, A., Erdohelyi, A., et al. (2018). CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B Environ. 221, 349–361. doi:10.1016/j.apcatb.2017.09.022
Ren, J., Mebrahtu, C., van Koppen, L., Martinovic, F., Hofmann, J. P., Hensen, E. J. M., et al. (2021). Enhanced CO2 methanation activity over La2-xCexNiO4 perovskite-derived catalysts: Understanding the structure-performance relationships. Chem. Eng. J. 426, 131760. doi:10.1016/j.cej.2021.131760
Riani, P., Valsamakis, I., Cavattoni, T., Sanchez Escribano, V., Busca, G., Garbarino, G., et al. (2021). Ni/SiO2-Al2O3 catalysts for CO2 methanation: Effect of La2O3 addition. Appl. Catal. B Environ. 284, 119697. doi:10.1016/j.apcatb.2020.119697
Rivas, I., Alvarez, J., Pietri, E., Pérez-Zurita, M. J., and Goldwasser, M. R. (2010). Perovskite-type oxides in methane dry reforming: Effect of their incorporation into a mesoporous SBA-15 silica-host. Catal. Today 149 (3-4), 388–393. doi:10.1016/j.cattod.2009.05.028
Rivas, M. E., Fierro, J. L. G., Goldwasser, M. R., Pietri, E., Pérez-Zurita, M. J., Griboval-Constant, A., et al. (2008). Structural features and performance of LaNi1−xRhxO3 system for the dry reforming of methane. Appl. Catal. A General 344 (1-2), 10–19. doi:10.1016/j.apcata.2008.03.023
Rodriguez, J. A., Grinter, D. C., Liu, Z., Palomino, R. M., and Senanayake, S. D. (2017). Ceria-based model catalysts: Fundamental studies on the importance of the metal-ceria interface in CO oxidation, the water-gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 46 (7), 1824–1841. doi:10.1039/c6cs00863a
Rosid, S. J. M., Toemen, S., Iqbal, M. M. A., Bakar, W., Mokhtar, W., Aziz, M. M. A., et al. (2019). Overview performance of lanthanide oxide catalysts in methanation reaction for natural gas production. Environ. Sci. Pollut. Res. 26 (36), 36124–36140. doi:10.1007/s11356-019-06607-8
Roy, S., Cherevotan, A., and Peter, S. C. (2018). Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges. ACS Energy Lett. 3 (8), 1938–1966. doi:10.1021/acsenergylett.8b00740
Ruocco, C., de Caprariis, B., Palma, V., Petrullo, A., Ricca, A., Scarsella, M., et al. (2019). Methane dry reforming on Ru perovskites, AZrRuO3: Influence of preparation method and substitution of A cation with alkaline Earth metals. J. CO2 Util. 30, 222–231. doi:10.1016/j.jcou.2019.02.009
Sadykov, V., Rogov, V., Ermakova, E., Arendarsky, D., Mezentseva, N., Alikina, G., et al. (2013). Mechanism of CH4 dry reforming by pulse microcalorimetry: Metal nanoparticles on perovskite/fluorite supports with high oxygen mobility. Thermochim. Acta 567, 27–34. doi:10.1016/j.tca.2013.01.034
Sastre, D., Serrano, D. P., Pizarro, P., and Coronado, J. M. (2019). Chemical insights on the activity of La1-xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 31, 16–26. doi:10.1016/j.jcou.2019.02.013
Senderens, J. B., and Sabatier, P. (1902). Nouvelles synthèses du méthane. Comptes Rendus Acad. Sci. 82, 514–516.
Shahnazi, A., and Firoozi, S. (2021). Improving the catalytic performance of LaNiO3 perovskite by manganese substitution via ultrasonic spray pyrolysis for dry reforming of methane. J. CO2 Util. 45, 101455. doi:10.1016/j.jcou.2021.101455
Shi, C., Wang, S., Ge, X., Deng, S., Chen, B., Shen, J., et al. (2021). A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 46, 101462. doi:10.1016/j.jcou.2021.101462
Shin, H. H., Lu, L., Yang, Z., Kiely, C. J., and McIntosh, S. (2016). Cobalt catalysts decorated with platinum atoms supported on barium zirconate provide enhanced activity and selectivity for CO2 methanation. ACS Catal. 6 (5), 2811–2818. doi:10.1021/acscatal.6b00005
Silva, C. K. S., Baston, E. P., Melgar, L. Z., and Bellido, J. D. A. (2019). Ni/Al2O3-La2O3 catalysts synthesized by a one-step polymerization method applied to the dry reforming of methane: Effect of precursor structures of nickel, perovskite and spinel. Reac. Kinet. Mech. Cat. 128 (1), 251–269. doi:10.1007/s11144-019-01644-3
Sreedhar, I., Varun, Y., Singh, S. A., Venugopal, A., and Reddy, B. M. (2019). Developmental trends in CO2 methanation using various catalysts. Catal. Sci. Technol. 9 (17), 4478–4504. doi:10.1039/c9cy01234f
Steiger, P., Kröcher, O., and Ferri, D. (2020). Increased nickel exsolution from LaFe0.8Ni0.2O3 perovskite-derived CO2 methanation catalysts through strontium doping. Appl. Catal. A General 590, 117328. doi:10.1016/j.apcata.2019.117328
Su, X., Xu, J., Liang, B., Duan, H., Hou, B., Huang, Y., et al. (2016). Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 25 (4), 553–565. doi:10.1016/j.jechem.2016.03.009
Su, Y.-J., Pan, K.-L., and Chang, M.-B. (2014). Modifying perovskite-type oxide catalyst LaNiO3 with Ce for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 39 (10), 4917–4925. doi:10.1016/j.ijhydene.2014.01.077
Sun, Z., Cai, T., Russell, C. K., Johnson, J. K., Ye, R.-P., Xiang, W., et al. (2020). Highly efficient methane decomposition to H2 and CO2 reduction to CO via redox looping of Ca2FexAl2-xO5 supported NiyFe3-yO4 nanoparticles. Appl. Catal. B Environ. 271, 118938. doi:10.1016/j.apcatb.2020.118938
Tabish, A., Varghese, A. M., Wahab, M. A., and Karanikolos, G. N. (2020). Perovskites in the energy grid and CO2 conversion: Current context and future directions. Catalysts 10 (1), 95. doi:10.3390/catal10010095
Talanov, M. V., and Talanov, V. M. (2021). Structural diversity of ordered pyrochlores. Chem. Mat. 33 (8), 2706–2725. doi:10.1021/acs.chemmater.0c04864
Tang, Y., Kobayashi, Y., Tassel, C., Yamamoto, T., and Kageyama, H. (2018). Hydride-enhanced CO2 methanation: Water-stable BaTiO2.4H0.6 as a new support. Adv. Energy Mat. 8 (23), 1800800. doi:10.1002/aenm.201800800
Tavasoli, A., and Ozin, G. (2018). Green syngas by solar dry reforming. Joule 2 (4), 571–575. doi:10.1016/j.joule.2018.02.017
Thalinger, R., Gocyla, M., Heggen, M., Dunin-Borkowski, R., Grünbacher, M., Stöger-Pollach, M., et al. (2016a). Ni–perovskite interaction and its structural and catalytic consequences in methane steam reforming and methanation reactions. J. Catal. 337, 26–35. doi:10.1016/j.jcat.2016.01.020
Thalinger, R., Götsch, T., Zhuo, C., Hetaba, W., Wallisch, W., Stöger-Pollach, M., et al. (2016b). Rhodium-catalyzed methanation and methane steam reforming reactions on rhodium-perovskite systems: Metal-support interaction. ChemCatChem 8 (12), 2057–2067. doi:10.1002/cctc.201600262
Thalinger, R., Opitz, A. K., Kogler, S., Heggen, M., Stroppa, D., Schmidmair, D., et al. (2015). Water-gas shift and methane reactivity on reducible perovskite-type oxides. J. Phys. Chem. C 119 (21), 11739–11753. doi:10.1021/acs.jpcc.5b02947
Tian, M., Wang, X. D., and Zhang, T. (2016). Hexaaluminates: A review of the structure, synthesis and catalytic performance. Catal. Sci. Technol. 6 (7), 1984–2004. doi:10.1039/c5cy02077h
Trump, B. A., Koohpayeh, S. M., Livi, K. J. T., Wen, J. J., Arpino, K. E., Ramasse, Q. M., et al. (2018). Universal geometric frustration in pyrochlores. Nat. Commun. 9 (1), 2619. doi:10.1038/s41467-018-05033-7
Tsiotsias, A. I., Charisiou, N. D., Yentekakis, I. V., and Goula, M. A. (2020). The role of alkali and alkaline earth metals in the CO2 methanation reaction and the combined capture and methanation of CO2. Catalysts 10 (7), 812. doi:10.3390/catal10070812
Tsounis, C., Wang, Y., Arandiyan, H., Wong, R. J., Toe, C. Y., Amal, R., et al. (2020). Tuning the selectivity of LaNiO3 perovskites for CO2 hydrogenation through potassium substitution. Catalysts 10 (4), 409. doi:10.3390/catal10040409
Ulmer, U., Dingle, T., Duchesne, P. N., Morris, R. H., Tavasoli, A., Wood, T., et al. (2019). Fundamentals and applications of photocatalytic CO2 methanation. Nat. Commun. 10 (1), 3169. doi:10.1038/s41467-019-10996-2
Utsis, N., Vidruk-Nehemya, R., Landau, M. V., and Herskowitz, M. (2016). Novel bifunctional catalysts based on crystalline multi-oxide matrices containing iron ions for CO2 hydrogenation to liquid fuels and chemicals. Faraday Discuss. 188, 545–563. doi:10.1039/c5fd00174a
Valderrama, G., Goldwasser, M. R., Navarro, C. U. d., Tatibouët, J. M., Barrault, J., Batiot-Dupeyrat, C., et al. (2005). Dry reforming of methane over Ni perovskite type oxides. Catal. Today 107-108, 785–791. doi:10.1016/j.cattod.2005.07.010
Valderrama, G., Kiennemann, A., de Navarro, C. U., and Goldwasser, M. R. (2018). LaNi1-xMnxO3 perovskite-type oxides as catalysts precursors for dry reforming of methane. Appl. Catal. A General 565, 26–33. doi:10.1016/j.apcata.2018.07.039
Valderrama, G., Urbina de Navarro, C., and Goldwasser, M. R. (2013). CO2 reforming of CH4 over Co–La-based perovskite-type catalyst precursors. J. Power Sources 234, 31–37. doi:10.1016/j.jpowsour.2013.01.142
Vignieri, S. (2020). Legacy of the disposable cup. Science 368 (6489), 383. doi:10.1126/science.368.6489.382-f
Wang, F., Wei, M., Evans, D. G., and Duan, X. (2016). CeO2-based heterogeneous catalysts toward catalytic conversion of CO2. J. Mat. Chem. A Mat. 4 (16), 5773–5783. doi:10.1039/c5ta10737g
Wang, H., Dong, X., Zhao, T., Yu, H., and Li, M. (2019). Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1-x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 245, 302–313. doi:10.1016/j.apcatb.2018.12.072
Wang, K., Han, C., Shao, Z., Qiu, J., Wang, S., Liu, S., et al. (2021). Perovskite oxide catalysts for advanced oxidation reactions. Adv. Funct. Mat. 31 (30), 2102089. doi:10.1002/adfm.202102089
Wang, M., Zhao, T., Dong, X., Li, M., and Wang, H. (2018). Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 224, 214–221. doi:10.1016/j.apcatb.2017.10.022
Wang, N., Yu, X., Wang, Y., Chu, W., and Liu, M. (2013). A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier. Catal. Today 212, 98–107. doi:10.1016/j.cattod.2012.07.022
Wang, X., Wei, B., Huang, X., Fan, M., Wang, Y., Chen, X., et al. (2020). Enhanced near-zero-CO2-emission chemicals-oriented oil production from coal with inherent CO2 recycling: Part I—PRB coal fast pyrolysis coupled with CO2/CH4 reforming. Int. J. Coal Sci. Technol. 7 (3), 433–443. doi:10.1007/s40789-020-00359-4
Wang, X., Ye, R., Duyar, M. S., Price, C. A. H., Tian, H., Chen, Y., et al. (2021). Design of mesoporous ZnCoSiOx hollow nanoreactors with specific spatial distribution of metal species for selective CO2 hydrogenation. Nano Res. 1–9. doi:10.1007/s12274-021-4013-8
Wang, X., Zhu, L., Zhuo, Y., Zhu, Y., and Wang, S. (2019). Enhancement of CO2 methanation over La-modified Ni/SBA-15 catalysts prepared by different doping methods. ACS Sustain. Chem. Eng. 7 (17), 14647–14660. doi:10.1021/acssuschemeng.9b02563
Wei, T., Jia, L., Luo, J.-L., Chi, B., Pu, J., Li, J., et al. (2020). CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 506, 144699. doi:10.1016/j.apsusc.2019.144699
Wu, J., Qiao, L.-Y., Zhou, Z.-F., Cui, G.-J., Zong, S.-S., Xu, D.-J., et al. (2018). Revealing the synergistic effects of Rh and substituted La2B2O7 (B = Zr or Ti) for preserving the reactivity of catalyst in dry reforming of methane. ACS Catal. 9 (2), 932–945. doi:10.1021/acscatal.8b03319
Wu, X., Xu, L., Chen, M., Lv, C., Wen, X., Cui, Y., et al. (2020). Recent progresses in the design and fabrication of highly efficient Ni-based catalysts with advanced catalytic activity and enhanced anti-coke performance toward CO2 reforming of methane. Front. Chem. 8, 581923. doi:10.3389/fchem.2020.581923
Xu, D.-J., Qiao, L.-Y., Zhou, Z.-F., Wu, J., Ye, R.-P., Qin, Y.-Y., et al. (2022). The exquisite inserting way: Pd and perovskite on the preferential oxidation of CO or H2. Mol. Catal. 524, 112316. doi:10.1016/j.mcat.2022.112316
Xu, L., Wang, E., Hou, X., Chen, J., He, Z., Liang, T., et al. (2020). Effect of incorporation of nitrogen on calcium hexaaluminate. J. Eur. Ceram. Soc. 40 (15), 6155–6161. doi:10.1016/j.jeurceramsoc.2020.06.057
Xu, R., Min, L., Qi, Z., Zhang, X., Jian, J., Ji, Y., et al. (2020). Perovskite transparent conducting oxide for the design of a transparent, flexible, and self-powered perovskite photodetector. ACS Appl. Mat. Interfaces 12 (14), 16462–16468. doi:10.1021/acsami.0c01298
Yadav, P. K., and Das, T. (2019). Production of syngas from carbon dioxide reforming of methane by using LaNixFe1−xO3 perovskite type catalysts. Int. J. Hydrogen Energy 44 (3), 1659–1670. doi:10.1016/j.ijhydene.2018.11.108
Yafarova, L. V., Chislova, I. V., Zvereva, I. A., Kryuchkova, T. A., Kost, V. V., Sheshko, T. F., et al. (2019). Sol–gel synthesis and investigation of catalysts on the basis of perovskite-type oxides GdMO3 (M = Fe, Co). J. Solgel. Sci. Technol. 92 (2), 264–272. doi:10.1007/s10971-019-05013-3
Yang, E.-h., Noh, Y.-s., Ramesh, S., Lim, S. S., and Moon, D. J. (2015). The effect of promoters in La0.9M0.1Ni0.5Fe0.5O3 (M = Sr, Ca) perovskite catalysts on dry reforming of methane. Fuel Process. Technol. 134, 404–413. doi:10.1016/j.fuproc.2015.02.023
Yang, E.-h., Noh, Y. S., Hong, G. H., and Moon, D. J. (2018). Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal. Today 299, 242–250. doi:10.1016/j.cattod.2017.03.050
Yang, L., Pastor-Pérez, L., Villora-Pico, J. J., Gu, S., Sepúlveda-Escribano, A., Reina, T. R., et al. (2020). CO2 valorisation via reverse water-gas shift reaction using promoted Fe/CeO2-Al2O3 catalysts: Showcasing the potential of advanced catalysts to explore new processes design. Appl. Catal. A General 593, 117442. doi:10.1016/j.apcata.2020.117442
Ye, R. P., Ding, J., Gong, W., Argyle, M. D., Zhong, Q., Wang, Y., et al. (2019a). CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 10 (1), 5698. doi:10.1038/s41467-019-13638-9
Ye, R. P., Gong, W., Sun, Z., Sheng, Q., Shi, X., Wang, T., et al. (2019b). Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 188, 116059. doi:10.1016/j.energy.2019.116059
Ye, R. P., Li, Q., Gong, W., Wang, T., Razink, J. J., Lin, L., et al. (2020). High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 268, 118474. doi:10.1016/j.apcatb.2019.118474
Yin, W. J., Weng, B., Ge, J., Sun, Q., Li, Z., Yan, Y., et al. (2019). Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 12 (2), 442–462. doi:10.1039/c8ee01574k
Zhan, H., Li, F., Gao, P., Zhao, N., Xiao, F., Wei, W., et al. (2014). Influence of element doping on La-Mn-Cu-O based perovskite precursors for methanol synthesis from CO2/H2. RSC Adv. 4, 48888–48896. doi:10.1039/c4ra07692c
Zhang, J., Ren, B., Fan, G., Yang, L., and Li, F. (2021). Exceptional low-temperature activity of a perovskite-type AlCeO3 solid solution-supported Ni-based nanocatalyst towards CO2 methanation. Catal. Sci. Technol. 11 (11), 3894–3904. doi:10.1039/d1cy00340b
Zhang, T., and Liu, Q. (2020a). Mesostructured cellular foam silica supported bimetallic LaNi1-xCoxO3 catalyst for CO2 methanation. Int. J. Hydrogen Energy 45 (7), 4417–4426. doi:10.1016/j.ijhydene.2019.12.006
Zhang, T., and Liu, Q. (2020b). Perovskite LaNiO3 nanocrystals inside mesostructured cellular foam silica: High catalytic activity and stability for CO2 methanation. Energy Technol. 8 (3), 1901164. doi:10.1002/ente.201901164
Zhang, Z., Zhao, G., Bi, G., Guo, Y., and Xie, J. (2021). Monolithic SiC-foam supported Ni-La2O3 composites for dry reforming of methane with enhanced carbon resistance. Fuel Process. Technol. 212, 106627. doi:10.1016/j.fuproc.2020.106627
Zhao, B., Yan, B., Jiang, Z., Yao, S., Liu, Z., Wu, Q., et al. (2018). High selectivity of CO2 hydrogenation to CO by controlling the valence state of nickel using perovskite. Chem. Commun. 54 (53), 7354–7357. doi:10.1039/c8cc03829e
Zhao, L., Chen, W., Li, Q., and Wu, W. (2021). Clean-energy utilization technology in the transformation of existing urban residences in China. Int. J. Coal Sci. Technol. 8 (5), 1138–1148. doi:10.1007/s40789-021-00417-5
Zheng, X.-G., Tan, S.-Y., Dong, L.-C., Li, S.-B., Chen, H.-M., Wei, S.-A., et al. (2015). Experimental and kinetic investigation of the plasma catalytic dry reforming of methane over perovskite LaNiO3 nanoparticles. Fuel Process. Technol. 137, 250–258. doi:10.1016/j.fuproc.2015.02.003
Zhu, J., Li, H., Zhong, L., Xiao, P., Xu, X., Yang, X., et al. (2014). Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 4 (9), 2917–2940. doi:10.1021/cs500606g
Keywords: CO2 conversions, oxygen vacancies, dispersion of active metal, strong metal-support interactions, perovskite-type mixed oxide based catalysts
Citation: Wu J, Ye R, Xu D-J, Wan L, Reina TR, Sun H, Ni Y, Zhou Z-F and Deng X (2022) Emerging natural and tailored perovskite-type mixed oxides–based catalysts for CO2 conversions. Front. Chem. 10:961355. doi: 10.3389/fchem.2022.961355
Received: 04 June 2022; Accepted: 01 July 2022;
Published: 05 August 2022.
Edited by:
Dedong He, Kunming University of Science and Technology, ChinaReviewed by:
Hui Li, Shanghai Normal University, ChinaJinguo Wang, Shanghai University of Engineering Sciences, China
Copyright © 2022 Wu, Ye, Xu, Wan, Reina, Sun, Ni, Zhou and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Runping Ye, cnllQG5jdS5lZHUuY24=; Zhang-Feng Zhou, emZ6aG91QGZqaXJzbS5hYy5jbg==; Xiaonan Deng, eG5fZGVuZ0Bmb3htYWlsLmNvbQ==
 Juan Wu
Juan Wu Runping Ye
Runping Ye Dong-Jie Xu3
Dong-Jie Xu3 Tomas Ramirez Reina
Tomas Ramirez Reina