
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 20 November 2017
Sec. Inflammation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.01605
This article is part of the Research Topic Oxidants and Redox Signaling in Inflammation View all 12 articles
 Jingjing Han1,2,3†
Jingjing Han1,2,3† Shoubao Ma1,3†
Shoubao Ma1,3† Huanle Gong1†
Huanle Gong1† Shuangzhu Liu2,3
Shuangzhu Liu2,3 Lei Lei1,2,3
Lei Lei1,2,3 Bo Hu1,3
Bo Hu1,3 Yang Xu1,2,3
Yang Xu1,2,3 Haiyan Liu4*
Haiyan Liu4* Depei Wu1,2,3*
Depei Wu1,2,3*
Acute graft-versus-host disease (aGVHD) remains a clinical challenge and a major source of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Dimethyl fumarate (DMF), an activator of Nrf2, has been shown to have anti-inflammatory and immunomodulatory properties without significant immunosuppression. We therefore hypothesized that DMF could be potentially harnessed for the treatment of aGVHD with retention of graft-versus-tumor effect. In this study, we showed that DMF significantly inhibited alloreactive T cell responses in vitro in mixed lymphocyte reaction assay. Administration of DMF significantly alleviated the severity, histological damage, and the overall mortality of aGVHD in an MHC-mismatched aGVHD model. DMF administration reduced the activation and effector function of donor T cells in vitro and in vivo. In addition, DMF treatment upregulated antioxidant enzymes heme oxygenase-1 and glutathione S-transferase-α1 expressions. Furthermore, DMF treatment markedly increased the frequencies of Treg cells. Depletion of CD25+ cells in DMF recipients aggravated aGVHD mortality compared with IgG control recipients. DMF could promote Treg cell differentiation in a dose dependent manner by upregulating TGF-β expression in vitro. Most importantly, DMF administration preserved graft-versus-leukemia effect after bone marrow transplantation. In conclusion, our findings demonstrated DMF as a promising agent for the prevention of aGVHD after allo-HSCT.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has become a potential curative treatment for malignant hematological diseases (1). However, the success of an allo-HSCT is frequently limited by life-threatening complications, such as acute graft-versus-host disease (aGVHD) (1). aGVHD is a T cell-mediated disease which is caused by alloreactive donor T cells recognizing and attacking recipient target organs, such as the liver, lungs, intestines and skin (2). Various effector T subsets, Th1, Th2, and Th17, are involved in the pathogenesis of aGVHD (3). They particularly contribute to the initiation and development and of aGVHD, and have been considered as potential targets for the treatment and prevention of aGVHD (3). Currently, therapy of established aGVHD is still dependent on corticosteroids, despite their limited efficacy and considerable toxicity (2). Therefore, development of novel therapies will be critical for the prevention and treatment of aGVHD.
It is previously noticed that conditioning regimens including high-dose chemotherapy and radiation therapy generally result in the formation of reactive oxygen species (ROS) in allo-HSCT patients, which triggers inflammatory response and tissue injury, and plays an important role in the development of aGVHD (4–6). Therefore, appropriate control of oxidative stress, particularly ROS production, is crucial for effectively managing aGVHD. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is the “master regulator” of the antioxidant response. Upon exposure to ROS, Nrf2 translocates to the nucleus, binds to antioxidant response elements (AREs) in combination with hundreds of genes located in the promoter region to confer antioxidant protective effects (7). Therefore, it is proposed that Nrf2 activation can scavenge oxygen free radicals produced by conditioning regimens of allo-HSCT, then therefore, inhibit oxidative stress damage to organs and tissues.
Dimethyl fumarate (DMF) was first proposed by a German chemist, Walter Schweckendiek, in 1959, initially for the treatment of psoriasis (8). It was then developed as an oral capsule for the treatment of adults with relapsing forms of multiple sclerosis (MS) (trade name Tecfidera) on March 27, 2013 (9). The pharmacological properties of DMF include the activation of Nrf2-dependent antioxidant response and inhibition of NF-κB pathway (10). On the one hand, DMF activates Nrf2 and induces the expression of many antioxidant defense enzymes, such as the sentinel cytoprotectant heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1, and glutathione S-transferase (GST) (11, 12). On the other hand, Nrf2 activation can simultaneously inhibit the NF-κB signaling pathway, and consequently modulate inflammatory cytokines and chemokine production, such as IL-1, IL-2, IL-6, iNOS, IFN-γ, as well as CCL2 and CXCL10 (11, 13, 14). Moreover, DMF could inhibit Th1 polarization and promote Th2 differentiation (15, 16), inhibit the maturation and function of dendritic cells (DCs), as well as the subsequent DC-mediated Th1 and Th17 cell responses (17). Furthermore, DMF also suppresses CCL2-induced chemotaxis of human monocytes (18), inhibits lipopolysaccharide (LPS) induced proinflammatory cytokine production in macrophages (19). Therefore, through a combination of Nrf2 activation and NF-κB signaling inhibition, DMF has been shown to have anti-inflammatory, anti-oxidative, and immunomodulatory properties without significant immunosuppression.
Based on the immunomodulatory effect of DMF, we hypothesize that DMF could have the potential for the treatment of aGVHD with retention of graft-versus-tumor effect after allo-HSCT. In this study, by using murine models of aGVHD and GVL, we showed that Nrf2 activation by DMF treatment significantly reduced aGVHD without impairing GVL effect. The protective role of DMF in aGVHD was associated with increased donor Tregs and reduced T cells infiltration and activation in aGVHD target organs. Our findings suggest that DMF can be used for the prevention and treatment of aGVHD.
Female C57BL/6 (H-2b) and BALB/C (H-2d) mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). All mice were maintained in a specific pathogen-free room at Animal Facilities of Soochow University. Experiments were carried out and approved according to the guidelines of the animal care and use committee at Soochow University. A20 lymphoma cells were purchased from American Type Culture Collection (Rockville, MD, USA). Cells were cultured at 37°C in a 5% CO2 incubator in RPMI 1640 culture media supplemented with 10% fetal bovine serum (Biological Industries, Co., Haemek, Israel). For bioluminescent imaging, stable luciferase-expressing A20 cells (A20-luc) were generated in our laboratory.
Murine aGVHD and GVL models were induced as described previously (20, 21). Briefly, BALB/c mice were given lethally 650 cGy (one dose) total body irradiation from X-ray, irradiated BALB/c mice was transplanted with 1 × 107 C57BL/6 bone marrow cells and 5 × 106 C57BL/6 spleen cells via the tail vein. DMF (30 mg/kg body weight, Item No. 50744, Sigma-Aldrich, USA) was administrated to the recipient mice by gavage once daily starting from day −3 to day 3 after bone marrow transplantation (BMT). 0.8% methocel (Sigma-Aldrich Fluka, USA) at the same volume was used as vehicle control. For GVL model, 1 × 106 A20-luc cells were added to bone marrow graft as mentioned above, and injected into lethally irradiated BALB/c mice. In vivo bioluminescence imaging was performed as described previously (20). Briefly, mice were given an intraperitoneal injection of 200 µg firefly luciferin and then anesthetized and imaged using Xenogen, IVIS 100 Bioluminescent Imaging System (Caliper Life Sciences, Hopkinton, MA, USA). Treg depletion was performed as described previously (22, 23). Briefly, lethally irradiated BALB/c mice were transplanted with 5 × 106 TCD-BM plus 1 × 106 total spleen T cells or CD25-depleted T cells from B6 mice, DMF was administered to these recipients, with vehicle treatment as control. T cell depletion was performed by anti-Thy1.2 mAb (30H12, Biolegend, USA) and rabbit complement (24). T cell purification was performed by using mouse T cell isolation kit (catalog #19851, Stemcell technologies, Vancouver, BC, USA), CD25 depletion was performed by using mouse CD25 regulatory T cell positive selection kit (catalog #18782, Stemcell technologies, USA) according to the manufacturer’s protocols. Unlabeled CD25 negative cells were collected. CD25 depletion efficiency was confirmed by FACS. The recipients were monitored daily for survival and every three days for body weight changes and clinical signs of GVHD. The severity of GVHD was assessed using a GVHD scoring system as described previously (20, 21).
Fourteen days after transplantation, liver, lung, small intestine and skin were obtained from the transplanted recipients and fixed in 10% formalin. Samples were then embedded in paraffin, sectioned were stained with hematoxylin and eosin. Tissue damage was assessed based on a semiquantitative scoring system as described previously (25, 26).
Mixed lymphocyte reaction assay was performed as described previously (27). Briefly, responder T cells were isolated from spleen of C57BL/6 mice by mouse T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). Stimulators were DCs from BALB/c cells. BM-derived DCs were generated and expanded from BALB/c mice with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) for 7 days. DCs were pretreated with DMF or DMSO for 24 h, then washed twice with PBS. 1 × 104 DCs treated as above were irradiated (30 Gy) and cocultured with 1 × 105 allogeneic T cells in U-bottom microwell plates. 5 days later, tritiated thymidine (3H-TdR, 1 mCi/well) (Shanghai Institute of Physics, Chinese Academy of Sciences) were added to the culture for 16–18 h prior to harvesting and were counted on a β-plate reader (PerkinElmer Instruments, Meriden, CT, USA). Cytokines in the supernatants were collected and measured by ELISA. In some experiments, T cells were labeled with CellTrace CFSE (5 µmol/L, Invitrogen) according to the manufacturer’s protocol. The CFSE dilution was examined by flow cytometry. For ex vivo MLR, splenocytes from transplanted recipients 14 days after BMT were as responders, irradiated splenocytes from BALB/c mice were as stimulators. Cytotoxicity assays were performed as described previously (20). Splenocytes from transplanted recipients 14 days after BMT were used as killing cells, and their killing ability of A20 targets was measured using CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Fitchburg, WI, USA).
The procedure for isolating single-cell suspensions from spleens has been described previously (21). Antibodies against CD3, CD4, CD8, CD11c, CD25, CD40, CD44, CD69, CD86, PD-1, PD-L1, IFN-γ, H-2kb, H-2kd used in this study were all purchased from BioLegend (San Diego, CA, USA). For cell surface staining, cell samples were stained with fluorescent dye-conjugated mAb for 20 min at 4°C in the presence of FcR-Block. For intracellular cytokine staining, cells were stimulated for 5 h with PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (10 µg/ml). Cells were harvested, washed, and stained with surface molecule antibodies in the presence of FcR-Block (eBioscience, San Diego, CA, USA). After the wash, cells were then fixed using CytoFix/CytoPerm buffer (BD Biosciences, USA) and stained with antibodies against intracellular cytokines or isotype control on ice for 30 min. Intracellular staining for FoxP3 was performed by using a Foxp3 staining kit (eBioscience, San Diego, CA, USA). Data were acquired on a NovoCyte Flow cytometer (ACEA Biosciences, San Diego, CA, USA) and analyzed using Flowjo software (FlowJo, Ashland, OR, USA).
Blood samples were obtained from recipients 14 days after BMT, serum was separated by centrifugation and was stored at −80°C. Culture supernatants were collected at indicated time by centrifugation. The levels of IL-2, IL-6, IFN-γ, TNF-α, TGF-β were examined by ELISA kit according to the manufacturer’s instructions (R&D system, Minneapolis, MN, USA).
For examining Nrf2 nuclear translocation, CD3+T cells were isolated from spleen of C57BL/6 mice and activated by plate bound anti-CD3 (5 µg/ml) and anti-CD28 (1 µg/ml) in the presence of DMF or DMSO for 3 h. The cells were harvested and fixed in 4% paraformaldehyde for 15 min, then permeabilized with 0.2% Triton X100 for 10 min, and blocked with 2% BSA for 30 min. Sample were incubated overnight with an anti-Nrf2 antibody (sc-13032, Santa Cruz, CA, USA) in 0.5% BSA. After three washes with PBS, cells were stained with Alexa Fluor 488 goat antirabbit IgG (Molecular Probes, USA). Cell nuclei were stained with DAPI. The fluorescent images were captured with the Leica DMi8 confocal microscope (Leica, Wetzlar, Germany).
Total RNA were extracted with TRIzol reagent (Takara, Japan) from aGVHD targets organs according to the manufacturer’s instructions. Transcription levels of Nrf2, Keap1, HO-1, GST-α1, IL-1β, IL-2, IL-6, IFN-γ genes were analyzed by real-time PCR using SYBR Green Master Mix (Applied Biosystems, Warrington, UK). The primers used were: Nrf2, Forward 5′-TAGATGACCATGAGTCGCTTGC-3′, reverse 5′-GCCAAACTTGCTCCATGTCC-3′; keap1, forward 5′-TGCCCCTGTGGTCAAAGTG-3′, reverse 5′-GGTTCGGTTACCGTCCTGC-3′; HO-1, forward 5′-AAGCCGAGAATGCTGAGTTCA-3′, reverse 5′-GCCGTGTAGATATGGTACAAGGA-3′; GST-α1, forward 5′-AAGCCCGTGCTTCACTACTTC-3′, reverse 5′-GGGCACTTGGTCAAACATCAAA-3′; β-actin, forward 5′-ATCTGGCACCACACCTTC-3′, reverse 5′-AGCCAGGTCCAGACGCA-3′. The relative expression of the gene was quantified using the comparative 2−ΔΔCt method relative to the housekeeping gene β-actin.
CD3+ T cells were isolated from spleen of C57BL/6 mice and activated by plate bound anti-CD3 (5 µg/ml) and anti-CD28 (1 µg/ml) in the presence of DMF or DMSO for 12 h. ROS levels were examined by Reactive Oxygen Species Assay Kit according to the manufacturer’s instructions (Beyotime, Shanghai, China). Briefly, cells were harvested and washed three times using RPMI-1640 media, 2′-7′-dichlorofluoresci1n diacetate was added at a final concentration of 10 µM to the cells. After a 20-min incubation at 37°C, cells were washed and resuspended in PBS. Data were acquired on a NovoCyte Flow cytometer and analyzed using Flowjo software.
For cell proliferation, 2 × 104 A20 cells were seeded into 96-well plates and treated with DMF or DMSO for 48 h. Then 10 µl of CCK-8 solution was added to each well and incubated for additional 2 h, the absorbance was measured at 450 nm. Cell apoptosis was evaluated using a Annexin V Apoptosis Detection Kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, 48 h after DMF treatment, the cells were washed with PBS and resuspended in 500 µl binding buffer with 5 µl annexin V and 10 µl propidium iodide. Data were acquired by flow cytometry and analyzed using Flowjo software.
Survival data were analyzed by log-rank test and Kaplan-Meier survival curves were generated using GraphPad Prism version 5 (GraphPad 6.0, San Diego, CA, USA). The data were expressed as the mean ± SD. Two-tailed Student’s t-test was used for statistical comparison between two groups. One-way ANOVA with Dunnet’s test was used for multiple comparisons. The significance levels are marked *P < 0.05; **P < 0.01; ***P < 0.001.
Dimethyl fumarate has been demonstrated to potently inhibit NF-κB activity while promote Nrf2 activation. Therefore, we investigated the impact of DMF on alloreactive T cell responses in MLR assays. BMDCs from BALB/c mice were cultured with allogeneic splenic CD3+T cells purified from C57BL/6 mice, in the presence of the Nrf2 activator DMF (Figure 1A). The results showed that DMF significantly inhibited the proliferation of alloreactive T cells in a dose-dependent manner on day 5 determined by 3H-TdR and CFSE dye dilution (Figures 1B,C). Consistent with the T cell proliferation results, cytokine analysis showed that proinflammatory cytokines IL-2, IL-6, IFN-γ, and TNF-α production were significantly decreased in a dose-dependent manner upon DMF treatment (Figure 1D). In addition, cell apoptosis assay showed that the apoptosis of alloreactive CD4+ T cells were significantly increased in the presence of DMF (Figures 1E,F). More interesting, the apoptotic cells were mainly CFSElow populations. To address whether DMF has a direct effect on T cells, we performed a T cell activation assay with anti-CD3/CD28 stimulation, the result showed that DMF could significantly inhibit T cells proliferation directly (Figure S1A in Supplementary Material). Additionally, we examined the effect of DMF on antigen presenting cells (APC). We cultured bone marrow DCs and then treated with DMF. The data showed that DMF had no effect on DC maturation, however, when DCs were preactivated by LPS, DMF could reduce CD80, CD86, and CD40 expression in a dose-dependent manner (Figure S1B–D in Supplementary Material). Similar results were observed in IL-6 and TNF-α expression (Figure S1E,F in Supplementary Material). Taken together, the results demonstrated that DMF could inhibit alloreactive T responses by suppressing cell proliferation and inducing cell apoptosis in vitro, through both direct effects on T cells and indirect effects on DCs.
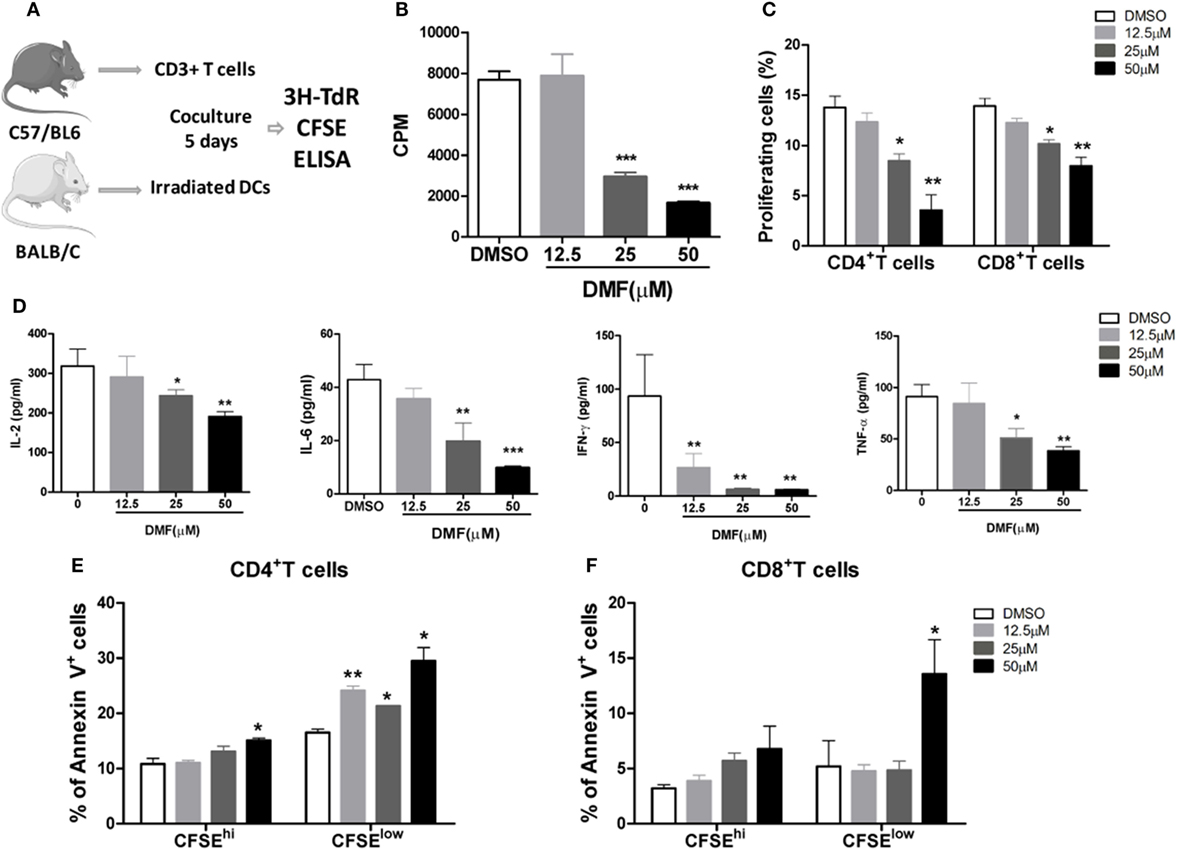
Figure 1. Dimethyl fumarate (DMF) inhibits alloreactive T cell responses in vitro. BMDCs from BALB/c mice were cocultured with allogeneic splenic CD3+ T cells purified from C57BL/6 mice, in the presence of the DMF (A), 5 days later, cell proliferation was evaluated by 3H-TdR (B) or CFSE (C). The levels of proinflammatory cytokines IL-2, IL-6, IFN-γ, and TNF-α in the supernatants were examined by ELISA (D). Cell apoptosis of alloreactive CD4+ T cells and CD8+ T cells was examined by Annexin V staining (E,F). Data shown are mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, compared with DMSO group (ANOVA with Dunnett’s test).
To determine the possible role of Nrf2 activation in aGVHD, we first examined the Nrf2 expression after allo-HSCT. We observed that Nrf2 mRNA levels were significantly decreased in the spleen, liver, lung and intestine of allogeneic BMT mice compared to those of syngeneic control animals (Figure 2A), suggesting that Nrf2 suppression could be involved in the pathogenesis of aGVHD. We then evaluated the potential impact of DMF on the aGVHD development in vivo by using a murine aGVHD model. As shown in Figure 2B, DMF treatment significantly prolonged survival (log rank, P = 0.005) of the hosts following allo-HSCT (Figure 2B). In addition, aGVHD scores were reduced in DMF-treated mice compared with vehicle control recipients (Figure 2C). Histological analysis revealed that there was decreased pathological damage in the liver, lung, intestine and skin of recipients receiving DMF 14 days after allo-HSCT (Figure 2D). Similar protective effect was observed when the dose of DMF was changed to 60 mg/kg from day −3 to day +3 post-allo-HSCT (data not shown). Moreover, DMF administration did not affect donor chimerism 14 days posttransplant (data not shown). Therefore, these data suggested that Nrf2 activation by DMF treatment in vivo significantly improved aGVHD outcomes as evidenced by prolonged survival and reduced aGVHD scores following allo-HSCT.
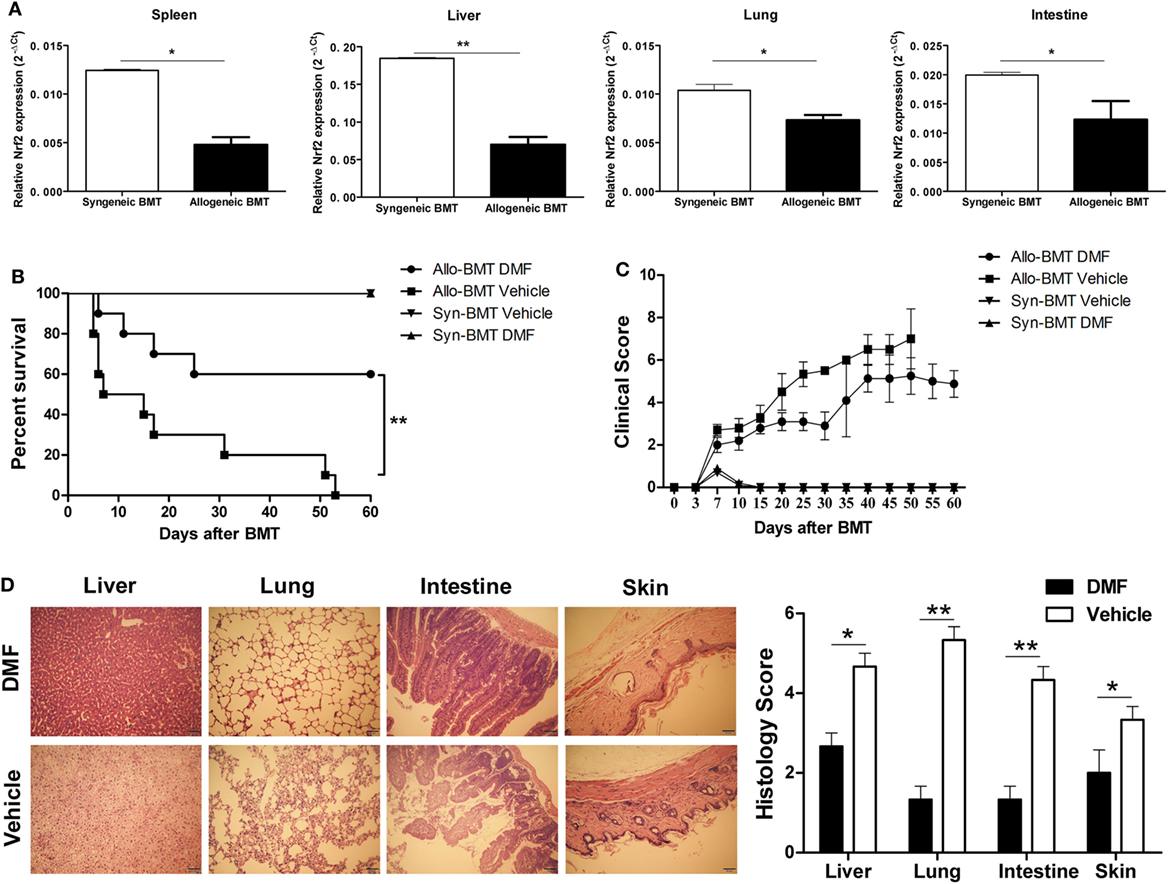
Figure 2. Dimethyl fumarate (DMF) administration alleviates acute graft-versus-host disease (aGVHD) in mice. Irradiated BALB/c mice was transplanted with 1 × 107 C57BL/6 bone marrow cells and 5 × 106 C57BL/6 spleen cells (allogeneic) or 1 × 107 BALB/c bone marrow cells and 5 × 106 BALB/c spleen cells (syngeneic). The mRNA level of Nrf2 in spleen, liver, lung and intestine of allogeneic or syngeneic bone marrow transplantation (BMT) mice was examined by qRT-PCR (A). DMF (30 mg/kg body weight) was administrated to the allogeneic recipient mice by gavage once daily starting from day −3 to day 3 after BMT. DMF treatment significantly prolongs survival (log rank, P = 0.005) (B) and reduced aGVHD severity (C) compared with vehicle control. Histological analysis revealed that there was decreased pathological damage in the liver, lung, intestine and skin of recipients receiving DMF 14 days after BMT (D). Survival data were analyzed by log-rank test and Kaplan-Meier survival curves were generated using GraphPad Prism. Data shown are mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, compared with vehicle group (Student’s t-test).
To investigate the potential mechanisms responsible for reduced aGVHD severity by DMF treatment, we analyzed the alloreactive T cell responses in vivo. On day 14 after allo-HSCT, decreased infiltration of donor CD3+ T and CD4+ T cells was observed in spleen of DMF-treated recipients compared with vehicle control recipients (Figure 3A). The ex vivo MLR assay further demonstrated that DMF treatment significantly reduced donor T cell alloreactivity (Figure 3B). In addition, both donor CD4+ T and CD8+ T cells had a reduced activation phenotype indicated by CD69 levels in the hosts treated with DMF (Figure 3C). Furthermore, purified CD4+ T cells were activated in vitro by anti-CD3/CD28, and DMF treatment significantly inhibited T cell activation and downregulated CD69, CD44, as well as PD-1 expressions. The CD25 levels, however, were slightly upregulated by DMF treatment (Figure 3D). Intracellular staining revealed that the frequencies of IFN-γ-producing donor CD4+ T and CD8+ T cells in spleen were significantly decreased in recipients given DMF (Figure 3E). Analysis of serum samples on day 14 following allo-HSCT showed that the levels of proinflammatory cytokines IL-6, IFN-γ as well as TNF-α were significantly downregulated in DMF-treated recipients compared with vehicle control recipients (Figure 3F).
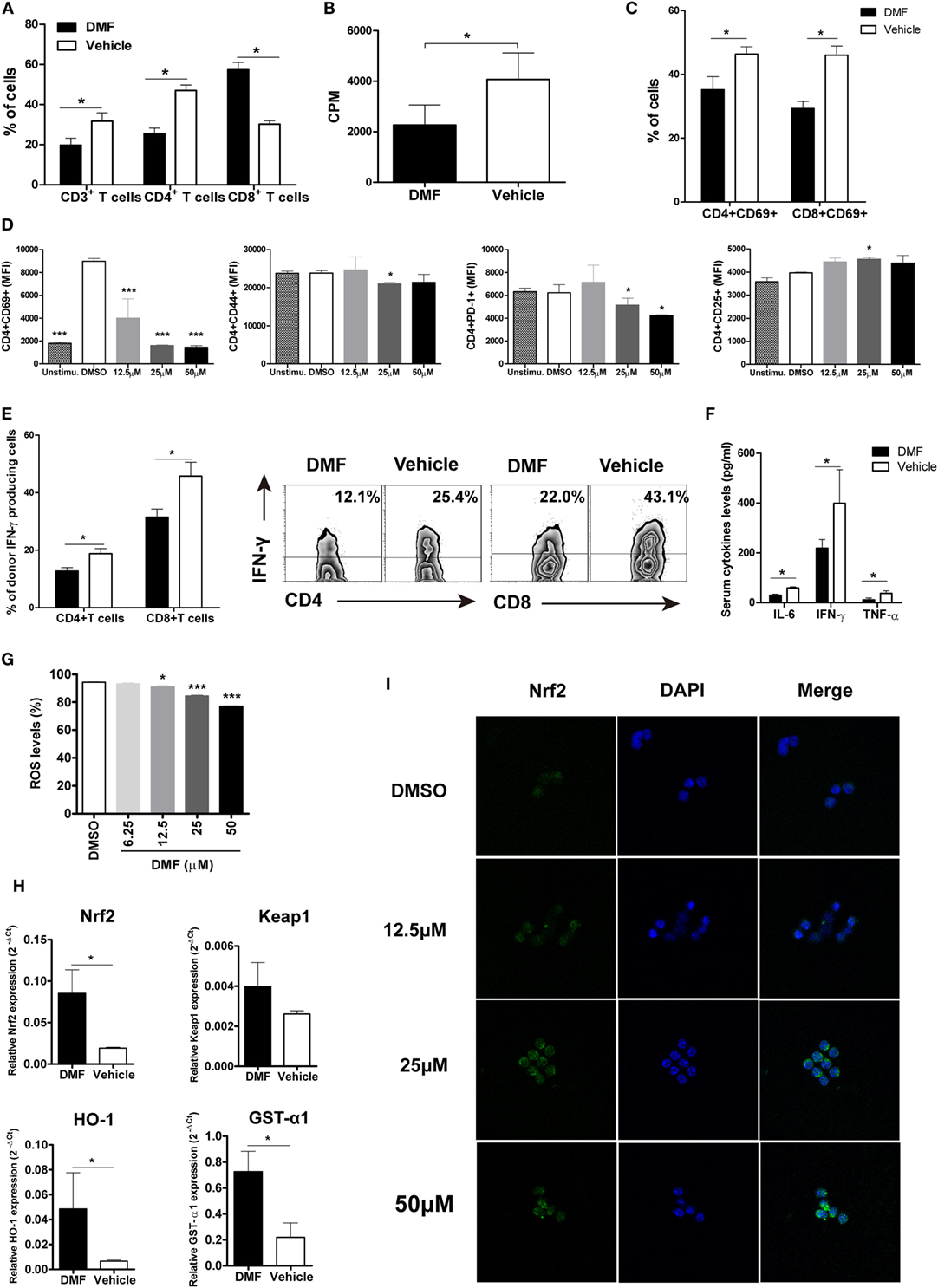
Figure 3. Dimethyl fumarate (DMF) administration reduces activation and effector function of donor T cells and upregulates antioxidant response. 14 days after bone marrow transplantation (BMT), the percentages of donor T cells in spleen were analyzed by FACS (A). Ex vivo mixed lymphocyte reaction (MLR) was performed by coculturing splenocytes from transplanted recipients 14 days after BMT with irradiated splenocytes from BALB/c mice. Cell proliferation was evaluated by 3H-TdR (B). CD69 expression on CD4+ and CD8+T cells of recipients mice 14 days after BMT was examined by FACS (C). Purified CD3+T cells were activated by anti-CD3/CD28 in vitro and treated with DMF. The activation markers CD69, CD44, CD25, and costimulatory molecule PD-1 levels were analyzed 24 h after treatment (D). IFN-γ production by CD4+ and CD8+T cells of recipients mice 14 days after BMT was examined by Intracellular staining (E). Serum levels of proinflammatory cytokines IL-6, IFN-γ, and TNF-α were examined by ELISA (F). Purified CD3+T were activated by anti-CD3/CD28 in vitro and treated with DMF for 12 h, reactive oxygen species (ROS) levels were examined by Reactive Oxygen Species Assay Kit (G). The Nrf2, keap 1, and antioxidant defense enzymes HO-1 and GST-α1 mRNA expressions in the spleen were examined by qRT-PCR 14 days after BMT (H). (I) CD3+T cells were isolated from spleen of C57BL/6 mice and activated by plate bound anti-CD3 (5 µg/ml) and anti-CD28 (1 µg/ml) in the presence of DMF or DMSO for 3 h. Nrf2 nuclear translocation was exmianed by immunofluorescent assay. Data shown are mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, compared with vehicle group (Student’s t-test), or DMSO group (ANOVA with Dunnett’s test).
Since DMF is a potent activator of Nrf2 and can induce the Nrf2-dependent antioxidant response, we then examined the ROS production and antioxidant genes expression. We found that DMF could significantly inhibit ROS production by activated T cells in a dose-dependent manner in vitro (Figure 3G). Moreover, the Nrf2 levels and antioxidant defense enzymes HO-1 and GST-α1 expressions were upregulated in recipients treated with DMF (Figure 3H). We then explored the effect of DMF on nuclear translocation of Nrf2, which has been demonstrated as a major mechanism of function for DMF. We observed a dose-dependent effect of DMF on nuclear translocation of Nrf2 in the CD3+ T cells by immunofluorescent staining (Figure 3I). Taken together, these results suggested that DMF could inhibit donor T cell alloreactivity, and production of proinflammatory cytokines, as well as upregulate antioxidant enzymes, which led to the alleviation of aGVHD severity.
Dimethyl fumarate upregulated CD25 expression on CD4 T cells, suggesting that DMF may promote the generation of Treg cells. We examined the frequencies and absolute numbers of CD4+Foxp3+ Treg cells in spleen on day 14 after allo-HSCT. As shown in Figures 4A,B, the frequencies, as well as the numbers of Treg cells in spleen were significantly increased in DMF-treated compared with vehicle control recipients, suggesting that DMF could increase Treg cells in vivo. In vitro MLR assay showed that DMF promoted Treg cell differentiation in a dose-dependent manner (Figure 4C). To further confirm that the effect of DMF on aGVHD was partially dependent on the promotion of Treg cells, mice were injected with anti-CD25 antibody to deplete Treg cells in vivo. As shown in Figure 4D, depletion of CD25+ cells in DMF recipients aggravated aGVHD mortality compared with IgG control recipients, while it had no effect on vehicle treatment recipients. TGF-β has been known to promote Treg generation, we therefore examined the TGF-β levels in serum of GVHD mice and in MLR culture supernatants. Date shown that TGF-β was increased in DMF recipients (Figure 4E) and upregulated upon DMF treatment in vitro (Figure 4F). Therefore, these results suggested that the protective effect of DMF treatment on aGVHD was at least partially dependent on the promotion of Treg cells.
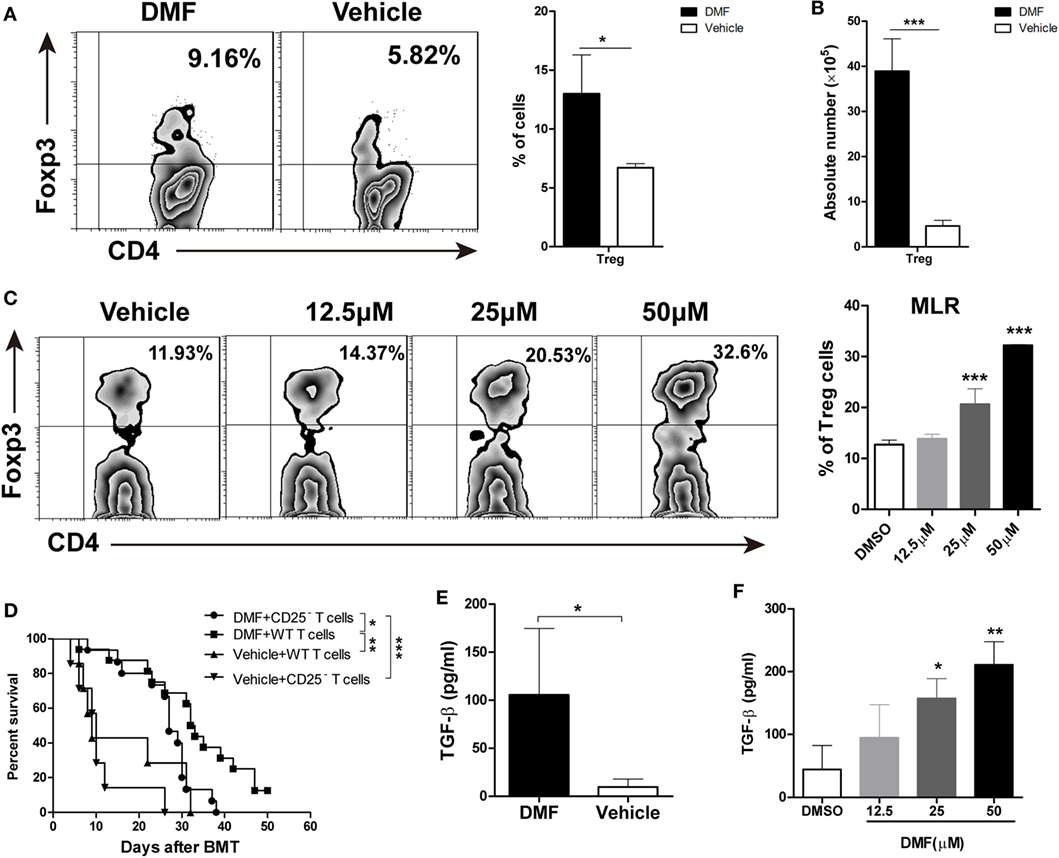
Figure 4. Dimethyl fumarate (DMF) inhibits acute graft-versus-host disease (aGVHD) by promoting Treg cells in vitro and in vivo. The frequencies and absolute number of CD4+Foxp3+ Treg cells in spleen on day 14 after bone marrow transplantation (BMT) were examined by FACS (A,B). Purified CD4+ T were activated by anti-CD3/CD28 in the presence of TGF-β (2 ng/ml) and IL-2 (50 U/ml) to induce Treg cells development in vitro, The effect of DMF on Treg cells differentiation was measured by FACS 4 days after Treg polarization (C). Lethally irradiated BALB/c mice were transplanted with 5 × 106 TCD-BM plus 1 × 106 total spleen T cells or CD25-depleted T cells from B6 mice. Depletion of CD25+ cells in DMF recipients aggravated aGVHD mortality compared with IgG control recipients (D). TGF-β levels in serum of aGVHD mice and MLR culture supernatants were measured by qRT-PCR (E,F). Survival data were analyzed by log-rank test and Kaplan-Meier survival curves were generated using GraphPad Prism. Data shown are mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, compared with DMSO group or vehicle group (Student’s t-test), or DMSO group (ANOVA with Dunnett’s test).
To evaluate the impact of DMF administration on GVL effects, aGVHD mice were challenged with A20-luc leukemia cells post-allo-HSCT. As shown in Figure 5A, mice transplanted with allo-BM alone plus A20-luc leukemia cells all died from leukemia within 40 days after transplant, regardless of whether DMF was administered. It has been reported that DMF could inhibit cell proliferation and induce apoptosis in a number of malignant cell lines including myeloid and lymphoid leukemia cell lines. However, the results in Figure 5A suggested that DMF may not directly affect A20 cell growth in vivo. DMF administration to mice receiving allo-BM and splenocytes, plus A20-luc showed prolonged survival compared with mice receiving vehicle control (Figure 5B), and low tumor burden was observed in DMF recipients as shown in bioluminescence imaging (Figure 5C), indicating the presence of GVL effect in DMF treated mice. The cell proliferation and apoptosis assay showed that low concentrations of DMF could not affect tumor growth and apoptosis in vitro, suggesting that DMF treatment inhibits A20 cell growth only at high concentrations, which may not be reached in vivo (Figures 5D,E). We also observed that donor T cells from DMF-treated recipients showed comparable, or even increased CTL killing activity against A20 leukemia cells compared to vehicle controls (Figure 5F). These findings further supported the observations that DMF treatment could preserve GVL effect after allo-HSCT.
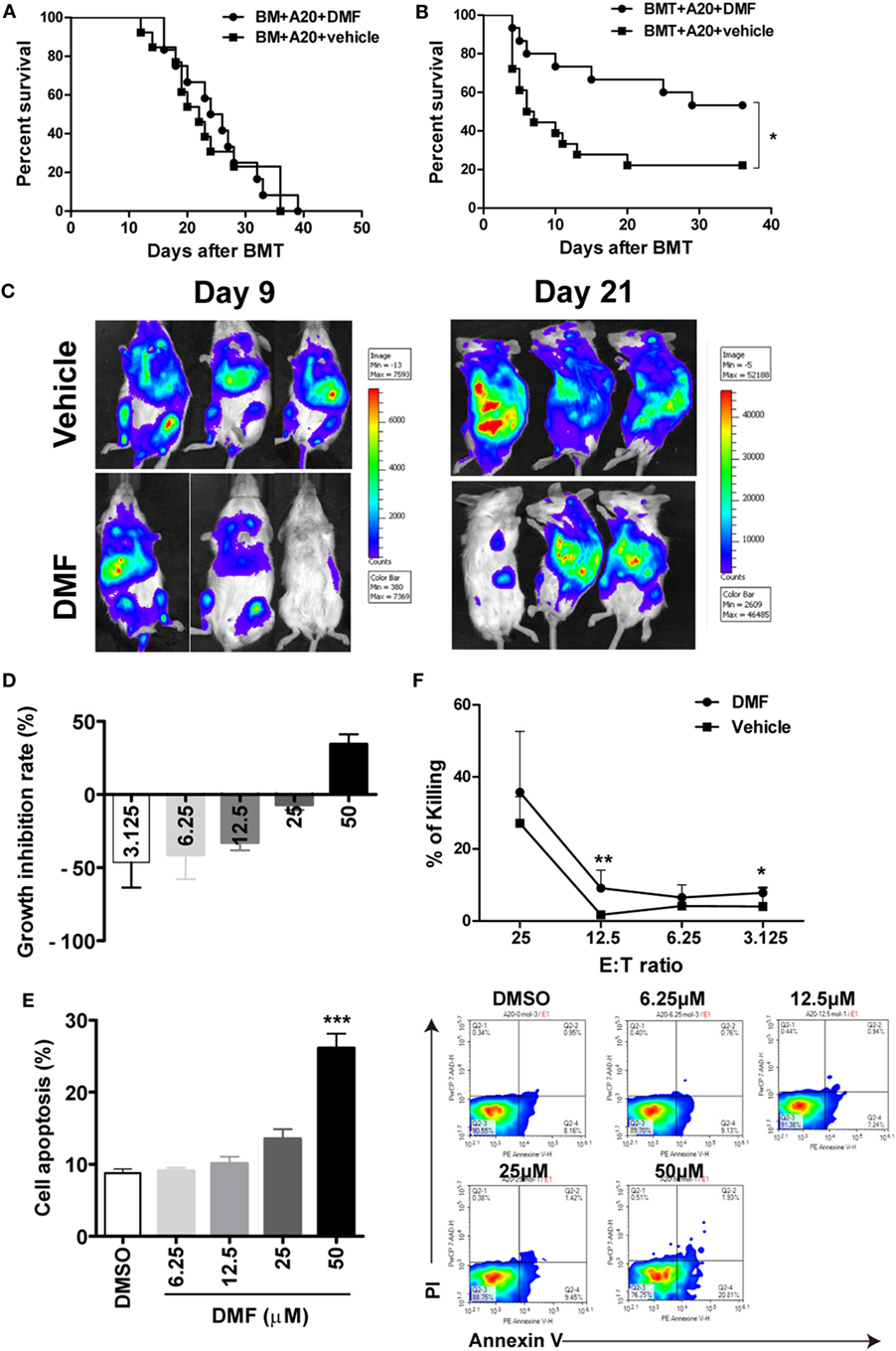
Figure 5. Dimethyl fumarate (DMF) administration preserves GVL effect after bone marrow transplantation (BMT). Mice were transplated with BM or BM and spleen plus A20-luc to establish GVL model. Mice transplanted with allo-BM alone plus A20-luc leukemia cells all died from leukemia regardless of whether DMF was administered (A). DMF administration to mice receiving allo-BMT plus A20-luc showed prolonged survival time and reduced tumor burden compared with mice receiving vehicle control (B,C). The effects of DMF on A20 cell proliferation (D) and apoptosis (E) were examined by CCK-8 assay and Annexin V/PI staining. Splenocytes from transplanted recipients 14 days after BMT were used as killing cells, and their killing ability of A20 targets was measured using CytoTox 96 nonradioactive cytotoxicity assay kit (F). Survival data were analyzed by log-rank test and Kaplan–Meier survival curves were generated using GraphPad Prism. Data shown are mean ± SD. *P < 0.05; **P < 0.01, compared with DMSO group (ANOVA with Dunnett’s test) or vehicle group (Student’s t-test).
Dimethyl fumarate has been shown to have potent anti-inflammatory or immunomodulatory properties without significant immunosuppression. It is effective in treating immune-mediated diseases, including psoriasis, MS as well as colitis (28–30). aGVHD is an immune-mediated disease which resulting from the activation of donor T lymphocytes by host antigen-presenting cell (APC) (2). During aGVHD, donor T cells activated by APCs express multiple immune effector molecules, such as fas ligand, perforin, IL-1β, IFN-γ, and TNF-α, leading to tissue damage (31, 32). We therefore hypothesize that DMF may be a new promising drug for the treatment of aGVHD.
In the present study, we first evaluated the effect of DMF on alloreactive T cell responses in MLR assays. We found that DMF significantly inhibited the proliferation and proinflammatory cytokine production of alloreactive T cells in a dose-dependent manner. In addition, DMF could also induce cell apoptosis of activated alloreactive T cells in vitro. In agreement with our results, Joachim et al., showed that DMF could inhibit lymphocyte proliferation and inflammatory cytokine secretion in human peripheral blood mononuclear cells (PBMCs) stimulated with LPS or lectin phytohemagglutinin or in human MLR assays (11). In addition, DMF could block IFN-γ- and LPS-induced Th1 chemokine (CXCL9 and CXCL10) production in a dose-dependent manner in PBMCs (33). DMF has also been shown to decrease adhesion molecule expression, such as CD25, HLA-DR and cutaneous lymphocyte-associated antigens (34). In our study, we also observed that DMF treatment significantly inhibited T cell activation and downregulated CD69, CD44, as well as PD-1 expressions, and IFN-γ production by donor CD4+ T and CD8+ T cells in spleen.
The maximum tolerated dose of DMF in human patients is 240 mg taken two to three times daily by oral for treatment of psoriasis (35), and MS (36). In preclinical studies, the effective and safe dose of DMF was 30 mg/kg in experimental autoimmune encephalomyelitis (37), experimental colitis (30) and tumor studies (38) in vivo. Therefore, based on the previous animal studies, we chose 30 mg/kg DMF in this study. We found, for the first time, that administration of DMF significantly ameliorated aGVHD in an MHC-mismatched BMT model. Interestingly, the administration window of DMF for treatment of aGVHD was −3 to +3 days post-BMT in our study, while the protective effect of DMF on aGVHD was not as effective when DMF was given from day 0 to day 7 (Data not shown), suggesting that DMF could regulate the sensitivity of the GVHD target organ to radiation induced injury. Administration of DMF pre-transplant is necessary to exert its protective effect. However, this notion still need further investigation.
Dimethyl fumarate protects the host from aGVHD may be via multiple mechanisms due to its dual role in Nrf2 pathway and NF-κB signaling. DMF treatment of T cells has previously been shown to decrease Th1 cytokine production, including IL-12, IFN-γ, TNF-α, and IL-17, and promote the expression of Th2 cytokines, such as IL-4 and IL-10 (10, 16, 39). In addition, DMF could also inhibit DCs maturation and subsequent DC mediated T cell responses (17). However, DMF metabolite monomethyl fumarate (MMF) can enhance CD56+ NK cells function by the upregulation of CD107a and granzyme B (40). In our murine aGVHD model, we found that DMF treatment reduced the proliferation and activation of donor T cells in spleen. Similarly, the IFN-γ expression by donor T cells, as well as the serum level of proinflammatory cytokines, was significantly decreased. Besides immune regulation, DMF also activates the Nrf2-dependent ARE pathway. We found that DMF could significantly inhibit ROS production in a dose-dependent manner in activated T cells. Moreover, the Nrf2 levels and antioxidant defense enzymes HO-1 and GST-α1 expressions were both unregulated in recipients given DMF. The ARE activation is involved in the induction of multiple downstream responses that protect cells from intracellular oxidative stress and injury, as well as modulate c cytokine and chemokine production (41). Therefore, our results suggest that DMF ameliorates aGVHD by modulating donor T cell activation and effector function, as well as upregulating antioxidant enzymes.
In our study, the activation makers on donor T cells were all downregulated upon DMF treatment except CD25. On the contrary, DMF significantly increased CD25 expression on activated T cells. Previous study showed that DMF promoted IL-2 secretion during human MLR (11). However, there is no evidence showing that DMF can regulate Treg cell development. We found that Treg cells were significantly increased in DMF-treated recipients compared with vehicle control in spleen on day 14 after allo-HSCT, accompanied by increased serum levels of TGF-β. In vitro MLR assay showed that DMF could promote Treg cell development in a dose-dependent manner. In addition, the depletion of Tregs in DMF recipients aggravated aGVHD mortality compared with IgG control recipients. Thus, promotion of Tregs cell development may be one of the mechanism that DMF inhibits aGVHD. Further studies are needed to explore the molecular mechanism of DMF in regulating Tregs development and function.
Alloreactive T-cells mediating aGVHD are also important for GVL activity, the ultimate goal of allo-HSCT is to separate GVHD from GVL effect. It has been suggested that GVL effect is primarily mediated by donor CD8+ T cells and NK cells, whereas CD4+ T cells mainly contribute to the development of aGVHD. In our study, we found that DMF administration did not weaken the GVL effect. Meanwhile, we did not observe inhibition of leukemia cell growth in vitro and in vivo by DMF treatment, suggesting that the antileukemia effect of DMF may be associated with its effect in maintaining cytolytic activity of donor CD8+ T cells and NK cells. To support this notion, cytotoxicity assay showed that CTLs from DMF-treated recipients has comparable, or even increased killing activity against leukemia cells compared to vehicle controls. Previous study has shown that DMF metabolite MMF can augment the NK cell lysis of K562 and RAJI leukemia cells through CD107a and Granzyme B (40). In addition, our results found that CD8+T cell numbers were not decreased upon DMF treatment, on the contrary, its proportions were significantly increased, which could be the reason for preserved GVL effects. Interestingly, it was reported that DMF treatment resulted in a preferential loss of CD8+ T cells compared with CD4+ T cells in patients with MS (42–46), while the proportions of Treg cells, circulating CD56(hi) NK cells, monocytes, and DCs were unaffected (43, 47). Although the underlying mechanisms remain largely unknown, recent data suggest that it maybe due to the differential susceptibility of distinct cell subsets to DMF-induced apoptosis (39). However, the differential effects of DMF on various immune cell subsets in different disease models need further investigation.
In conclusion, we provide evidence for the first time that the Nrf2 activator DMF reduces aGVHD with retention of GVL effect. DMF treatment promoted donor Treg development and reduced alloreactive T cells response, as well as upregulated antioxidant enzyme expressions. Our findings demonstrated DMF as a promising agent for the prevention of aGVHD after allo-HSCT.
All animal experiments were carried out and approved according to the guidelines of the animal care and use committee at Soochow University.
HL and DW designed the study; SM, JH, and HG performed the experiments; SL, LL, BH, and YX contributed to the experiments; SM analyzed the data; and SM, HL, and DW wrote the manuscript. All authors have discussed and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work has been supported by the grants from National Natural Science Foundation of China (81400145), Natural Science Foundation of Jiangsu Province (BK201500352), China Postdoctoral Science Foundation (7131702415). The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The Innovation Capability Development Project of Jiangsu Province (no. BM2015004). The National Key Research And Development Program (2016YFC0902800).
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fimmu.2017.01605/full#supplementary-material.
Figure S1. DMF inhibited T cells proliferation and DCs maturation in vitro. CD3+T cells were isolated from spleen of C57BL/6 mice and activated by plate bound anti-CD3 (5 µg/ml) and anti-CD28 (1 µg/ml) in the presence of DMF or DMSO for 48 h. cell proliferation was measured by 3H-TdR (A), Data shown are mean ± SD. ***P < 0.001, compared with DMSO group (ANOVA with Dunnett’s test). BM-derived DCs were generated and expanded from BALB/c mice with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml). DCs were treated with DMF or DMSO for 24 h in the present or absence of LPS (1 µg/ml), CD80, CD86, and CD40 on the DCs were examined by FACS (B–D). Cytokines IL-6, TNF-α, IFN-γ in the supernatants were collected and measured by ELISA (E–G). Data shown are mean ± SD. ***P < 0.001 compared with LPS group (ANOVA with Dunnett’s test).
1. Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J (2011) 1(4):e16. doi:10.1038/bcj.2011.14
2. Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol (2014) 11(9):536–47. doi:10.1038/nrclinonc.2014.102
3. Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood (2011) 117(12):3268–76. doi:10.1182/blood-2010-12-290403
4. Cetin T, Arpaci F, Yilmaz MI, Saglam K, Ozturk B, Komurcu S, et al. Oxidative stress in patients undergoing high-dose chemotherapy plus peripheral blood stem cell transplantation. Biol Trace Elem Res (2004) 97(3):237–47. doi:10.1385/BTER:97:3:237
5. Shen H, Yu H, Liang PH, Cheng H, XuFeng R, Yuan Y, et al. An acute negative bystander effect of gamma-irradiated recipients on transplanted hematopoietic stem cells. Blood (2012) 119(15):3629–37. doi:10.1182/blood-2011-08-373621
6. Tkachev V, Goodell S, Opipari AW, Hao LY, Franchi L, Glick GD, et al. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J Immunol (2015) 194(12):5789–800. doi:10.4049/jimmunol.1402180
7. Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog (2009) 48(2):91–104. doi:10.1002/mc.20465
8. Nieboer C, de Hoop D, van Loenen AC, Langendijk PN, van Dijk E. Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol (1989) 20(4):601–8. doi:10.1016/S0190-9622(89)70071-2
9. US Food and Drug Administration. FDA Approves New Multiple Sclerosis Treatment: Tecfidera. Silver Spring, MD: FDA gov (2013).
10. Gill AJ, Kolson DL. Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol (2013) 33(4):307–59. doi:10.1615/CritRevImmunol.2013007247
11. Lehmann JC, Listopad JJ, Rentzsch CU, Igney FH, von Bonin A, Hennekes HH, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol (2007) 127(4):835–45. doi:10.1038/sj.jid.5700686
12. Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther (2012) 341(1):274–84. doi:10.1124/jpet.111.190132
13. Gillard GO, Collette B, Anderson J, Chao J, Scannevin RH, Huss DJ, et al. DMF, but not other fumarates, inhibits NF-kappaB activity in vitro in an Nrf2-independent manner. J Neuroimmunol (2015) 283:74–85. doi:10.1016/j.jneuroim.2015.04.006
14. Liu X, Zhou W, Zhang X, Lu P, Du Q, Tao L, et al. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol (2016) 112:37–49. doi:10.1016/j.bcp.2016.05.002
15. Ockenfels HM, Schultewolter T, Ockenfels G, Funk R, Goos M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol (1998) 139(3):390–5. doi:10.1046/j.1365-2133.1998.02400.x
16. Ghoreschi K, Bruck J, Kellerer C, Deng C, Peng H, Rothfuss O, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med (2011) 208(11):2291–303. doi:10.1084/jem.20100977
17. Peng H, Guerau-de-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor kappaB (NF-kappaB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem (2012) 287(33):28017–26. doi:10.1074/jbc.M112.383380
18. Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol (2011) 187(10):5015–25. doi:10.4049/jimmunol.1101868
19. McGuire VA, Ruiz-Zorrilla Diez T, Emmerich CH, Strickson S, Ritorto MS, Sutavani RV, et al. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci Rep (2016) 6:31159. doi:10.1038/srep31159
20. Liu Y, Wu Y, Wang Y, Cai Y, Hu B, Bao G, et al. IL-35 mitigates murine acute graft-versus-host disease with retention of graft-versus-leukemia effects. Leukemia (2015) 29(4):939–46. doi:10.1038/leu.2014.310
21. Cai Y, Ma S, Liu Y, Gong H, Cheng Q, Hu B, et al. Adoptively transferred donor IL-17-producing CD4+ T cells augment, but IL-17 alleviates, acute graft-versus-host disease. Cell Mol Immunol (2016). doi:10.1038/cmi.2016.37
22. Saha A, O’Connor RS, Thangavelu G, Lovitch SB, Dandamudi DB, Wilson CB, et al. Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. J Clin Invest (2016) 126(7):2642–60. doi:10.1172/JCI85796
23. Long J, Chang L, Shen Y, Gao WH, Wu YN, Dou HB, et al. Valproic acid ameliorates graft-versus-host disease by downregulating Th1 and Th17 cells. J Immunol (2015) 195(4):1849–57. doi:10.4049/jimmunol.1500578
24. Hu B, Bao G, Zhang Y, Lin D, Wu Y, Wu D, et al. Donor NK cells and IL-15 promoted engraftment in nonmyeloablative allogeneic bone marrow transplantation. J Immunol (2012) 189(4):1661–70. doi:10.4049/jimmunol.1103199
25. Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J Jr, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest (1998) 102(10):1882–91. doi:10.1172/JCI4285
26. Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood (2005) 105(5):2227–34. doi:10.1182/blood-2004-08-3032
27. Liang Y, Ma S, Zhang Y, Wang Y, Cheng Q, Wu Y, et al. IL-1beta and TLR4 signaling are involved in the aggravated murine acute graft-versus-host disease caused by delayed bortezomib administration. J Immunol (2014) 192(3):1277–85. doi:10.4049/jimmunol.1203428
28. Sorensen PS, Sellebjerg F. Oral fumarate for relapsing-remitting multiple sclerosis. Lancet (2008) 372(9648):1447–8. doi:10.1016/S0140-6736(08)61605-0
29. Bovenschen HJ, Langewouters AM, van de Kerkhof PC. Dimethylfumarate for psoriasis: pronounced effects on lesional T-cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. Am J Clin Dermatol (2010) 11(5):343–50. doi:10.2165/11533240-000000000-00000
30. Casili G, Cordaro M, Impellizzeri D, Bruschetta G, Paterniti I, Cuzzocrea S, et al. Dimethyl fumarate reduces inflammatory responses in experimental colitis. J Crohns Colitis (2016) 10(4):472–83. doi:10.1093/ecco-jcc/jjv231
31. Liang Y, Mao X, Liu H. Proteasome inhibitor clioquinol as a candidate drug in prophylaxis and treatment of acute graft-versus-host disease. Med Hypotheses (2011) 76(3):400–2. doi:10.1016/j.mehy.2010.11.002
32. Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol (2006) 43(1):3–10. doi:10.1053/j.seminhematol.2005.09.001
33. Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol (2001) 144(6):1114–20. doi:10.1046/j.1365-2133.2001.04220.x
34. Rubant SA, Ludwig RJ, Diehl S, Hardt K, Kaufmann R, Pfeilschifter JM, et al. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J Invest Dermatol (2008) 128(2):326–31. doi:10.1038/sj.jid.5700996
35. Balak DM, Fallah Arani S, Hajdarbegovic E, Hagemans CA, Bramer WM, Thio HB, et al. Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. Br J Dermatol (2016) 175(2):250–62. doi:10.1111/bjd.14500
36. Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med (2012) 367(12):1087–97. doi:10.1056/NEJMoa1206328
37. Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest (2014) 124(5):2188–92. doi:10.1172/JCI72151
38. Nicolay JP, Muller-Decker K, Schroeder A, Brechmann M, Mobs M, Geraud C, et al. Dimethyl fumarate restores apoptosis sensitivity and inhibits tumor growth and metastasis in CTCL by targeting NF-kappaB. Blood (2016) 128(6):805–15. doi:10.1182/blood-2016-01-694117
39. Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y. Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol (2017) 198(8):3069–80. doi:10.4049/jimmunol.1601532
40. Vego H, Sand KL, Hoglund RA, Fallang LE, Gundersen G, Holmoy T, et al. Monomethyl fumarate augments NK cell lysis of tumor cells through degranulation and the upregulation of NKp46 and CD107a. Cell Mol Immunol (2016) 13(1):57–64. doi:10.1038/cmi.2014.114
41. Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res (2010) 44(11):1267–88. doi:10.3109/10715762.2010.507670
42. Fleischer V, Friedrich M, Rezk A, Buhler U, Witsch E, Uphaus T, et al. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult Scler (2017). doi:10.1177/1352458517703799
43. Gross CC, Schulte-Mecklenbeck A, Klinsing S, Posevitz-Fejfar A, Wiendl H, Klotz L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm (2016) 3(1):e183. doi:10.1212/NXI.0000000000000183
44. Berkovich R, Weiner LP. Effects of dimethyl fumarate on lymphocyte subsets. Mult Scler Relat Disord (2015) 4(4):339–41. doi:10.1016/j.msard.2015.06.002
45. Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm (2015) 2(3):e76. doi:10.1212/NXI.0000000000000076
46. Chaves C, Ganguly R, Ceresia C, Camac A. Lymphocyte subtypes in relapsing-remitting multiple sclerosis patients treated with dimethyl fumarate. Mult Scler J Exp Transl Clin (2017) 3(2):2055217317702933. doi:10.1177/2055217317702933
Keywords: acute graft-versus-host disease, graft-versus-leukemia, Nrf2, dimethyl fumarate, Treg cells
Citation: Han J, Ma S, Gong H, Liu S, Lei L, Hu B, Xu Y, Liu H and Wu D (2017) Inhibition of Acute Graft-versus-Host Disease with Retention of Graft-versus-Tumor Effects by Dimethyl Fumarate. Front. Immunol. 8:1605. doi: 10.3389/fimmu.2017.01605
Received: 25 July 2017; Accepted: 07 November 2017;
Published: 20 November 2017
Edited by:
Gabor Csanyi, Augusta University, United StatesReviewed by:
Natasha Mireille Rogers, University of Pittsburgh, United StatesCopyright: © 2017 Han, Ma, Gong, Liu, Lei, Hu, Xu, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Liu, bWljbGl1aEBudXMuZWR1LnNn;
Depei Wu, d3VkZXBlaUBtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.