Introduction: Various polymers have been investigated and developed to resist to non-specific adhesion to avoid or limit adsorption of proteins, cells, bacterial or platelets adhesion, known to lead to inflammation, infections and to be the main responsible for the eventual failure of the implant. Thus, a major challenge in the field aims to decrease the hydrophobicity for enhancing the biocompatibility in an effort for compromising biological and mechanical properties. Polysaccharides, polyacrylates and their derivatives have been reported in many biomedical fields as potential candidates for antifouling, tissue engineering or hemocompatible applications[1]-[3]. This work aimed to develop and investigate copolymers of carboxymethyldextran and polybutylmethacrylate which are susceptible to show interesting mechanical and non-adherent properties especially suitable for vascular applications.
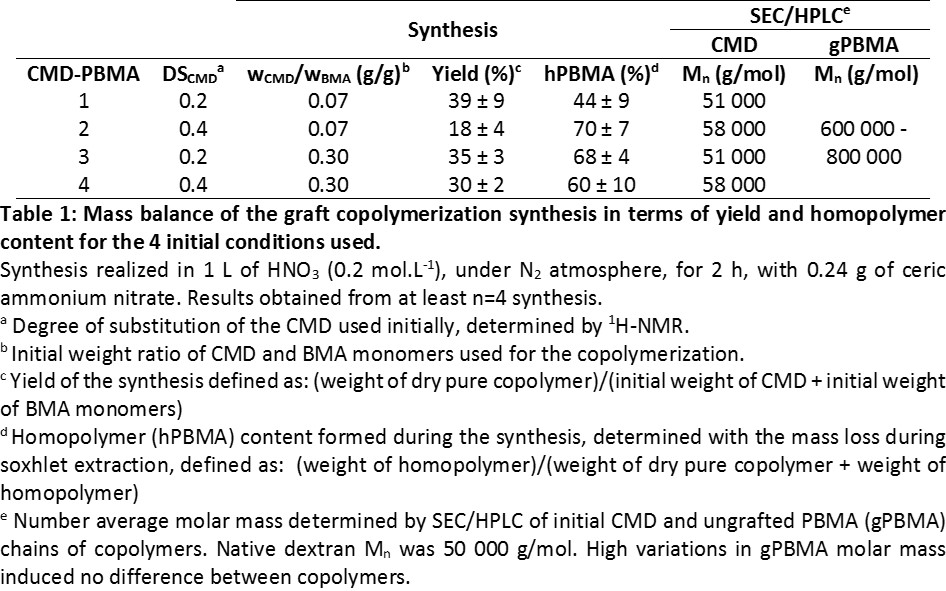
Material and Methods: Graft copolymers (CMD-PBMA) have been synthesized by radical copolymerization with Ce(IV) ions, from butyl methacrylate monomers (BMA) and carboxymethyldextran (CMD) (Table 1)[4],[5]. Synthesis were characterized by mass balance, and copolymers by solid state 13C-NMR, FTIR as well as by SEC/HPLC after acid hydrolysis allowing to differentiate PBMA and CMD chains. Films have been prepared by dispersion of CMD-PBMA (50 mg/mL) in THF/water (90:10) poured in a mold and placed under THF atmosphere with CaCl2 overnight. Relaxation assay (ɛ = 3 %) were performed on dumbbell-shaped films with an Instron 5944 system, at 10 mm/min, until 25% of deformation and then up to rupture. Cytotoxicity and proliferation assays were performed with 3T3 fibroblast (15000 cells/cm²) and HUVEC (40000 cell/cm²). Cytotoxicity was investigated after 24h by resazurin with 1 and 7 days extracts. Cells viability was tested with resazurin prior observation of proliferation by fluorescence microscopy after 1, 4 and 7 days. Clotting assay was evaluated after 25 min of contact with whole blood by measuring free hemoglobin in absorbance.
Results and Discussion: NMR and FTIR spectra of copolymers clearly evidenced the characteristic peaks of both CMD and PBMA. Whatever the copolymer considered, they were mostly made of PBMA chains (80-90%) which were 10 times larger than CMD ones as seen in Table 1. Furthermore, the reaction yield was quite low mainly due to PBMA homopolymer formation. It has been noticed that for both ratios of CMD/BMA used, CMD 0.2 led to higher yields than CMD 0.4, due to more available substitution sites (less COOH) for radical copolymerization. Latest observations, get along with the polymerization scheme involving PBMA macro-radicals formation and their recombination on the CMD backbone.
Despite these differences, all copolymers films showed equivalent composition after XPS analyses and higher hydrophilicity than PBMA (80° vs 95°). Mechanical relaxation assays on CMD-PBMA films showed quasi-total relaxation of stress and elongations to fracture up to 60% whereas PBMA films were too brittle to be unmolded. Copolymer films did not induce cytotoxicity and HUVECs and 3T3 fibroblasts did not adhere, therefore no further proliferation has been observed (Figure 1). No difference in hemocompatibility was observed between copolymers and PBMA.
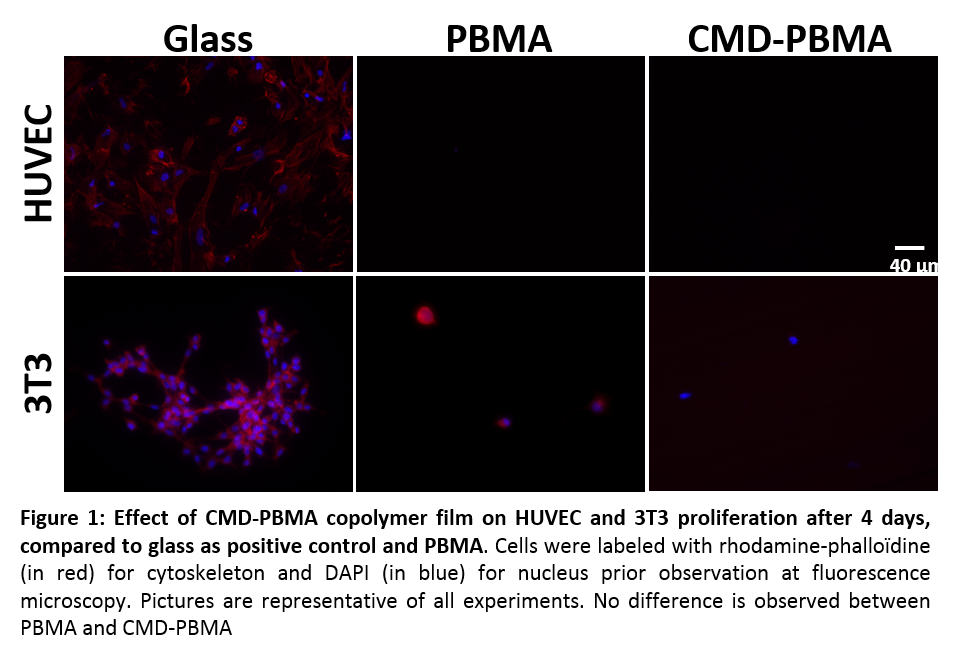
Conclusions: The presence of CMD in the copolymer lead to increase mechanical resistance while antifouling properties of PBMA are preserved, as no cells adhere, and non-cytotoxicity is shown. Regardless the substitution degree of CMD and the BMA/CMD ratio considered, copolymers exhibit similar biological and mechanical properties. Therefore, these copolymers appear as promising materials for non-adherent coatings or polymers for vascular applications.
This work was supported by Inserm-FRSQ, FP7 european program PRESTIGE, the Natural Science and Engineering Research Council of Canada, the Quebec Ministry for International Relations, the Centre Franco-Québecois de Coopération Universitaire, Centre d’Études et de Recherche sur les Matériaux Avancés (CERMA-ULaval), the CHU de Québec Research Center, the Centre Franco-Québecois de Coopération Universitaire, and the Research Center of CHU de Québec. The authors would like to express their gratitude to all the volunteers who donated blood and all technicians involved.
References:
[1] McLean, K. et al. Method of immobilization of carboxymethyl-dextran affects resistance to tissue and cell colonization. Colloids Surf. B. Biointerfaces 18, 221–234 (2000).
[2] Möller, S., Weisser, J., Bischoff, S. & Schnabelrauch, M. Dextran and hyaluronan methacrylate based hydrogels as matrices for soft tissue reconstruction. Biomol. Eng. 24, 496–504 (2007).
[3] Lee, J. H. & Oh, S. H. MMA/MPEOMA/VSA copolymer as a novel blood-compatible material: effect of PEO and negatively charged side chains on protein adsorption and platelet adhesion. J. Biomed. Mater. Res. 60, 44–52 (2002).
[4] Michel, E. C. et al. Dextran grafting on PTFE surface for cardiovascular applications. Biomatter 4, 1–10 (2014).
[5] Wang, L., Tu, K., Li, Y. & Zhang, J. Synthesis and characterization of temperature responsive graft copolymers of dextran with poly ( N-isopropylacrylamide). React. Funct. Polym. 53, 19–27 (2002).