Introduction: Mesenchymal stem cells (MSCs) hold promise to treat ischemic conditions. However, direct delivery of MSCs in vivo often resulted in low cell retention. Recent studies have shown that MSC spheroids provide microenvironment that can enhance cell-cell interaction and prolong the cell survival in vivo. Here, we aim to develop scaffold materials that can be used to improve the delivery and therapeutic outcome of MSCs in hindlimb ischemia (HLI). A microwell-patterend electrospun membrane was fabricated and loaded with MSC spheroids, and the ability of the system to maintain the cell survival and re-establish blood perfusion in ischemic mouse hindlimbs was evaluated.
Materials and Methods: The microwell-patterned scaffolds were prepared by electrospinning of a polyurethane onto a micro-patterned polydimethylsiloxane collector. HLI models were generated in 129 mice. MSC spheroids were generated on ultra-low attachment microplates. Scaffolds loaded with or without cells were subcutaneously implanted into the ischemic leg. Laser Doppler Perfusion Imaging (LDPI) was used to exam the recovery of blood flow. Lectin staining was performed on parafin sections of ischemic tissues to quantify the density of blood vessels. Bioluminescence (BLI) imaging was used to visualize cell retention.
Results and Discussion: MSC spheroids were located in the microwells and the scaffolds were effective to maintain the spheroid organization and enhance the MSC gene expression in vitro. Upon transplantion in vivo, the group of MSC spheroids with scaffolds had significantly higher perfusion levels from day 3 to day 14 in ischemic legs compared to the control groups including mice injected with single MSCs or MSC spheroids without scaffolds. Lectin staining showed that MSC spheroids transplanted with scaffolds induced a approximate 2-fold increase in blood vessel density compared to all control groups. In the BLI analysis using luciferase-transfected MSCs, while the injected single MSCs were undetectale within 7 days, the spheroids transplanted via patterend scaffolds survived up to 2 weeks.
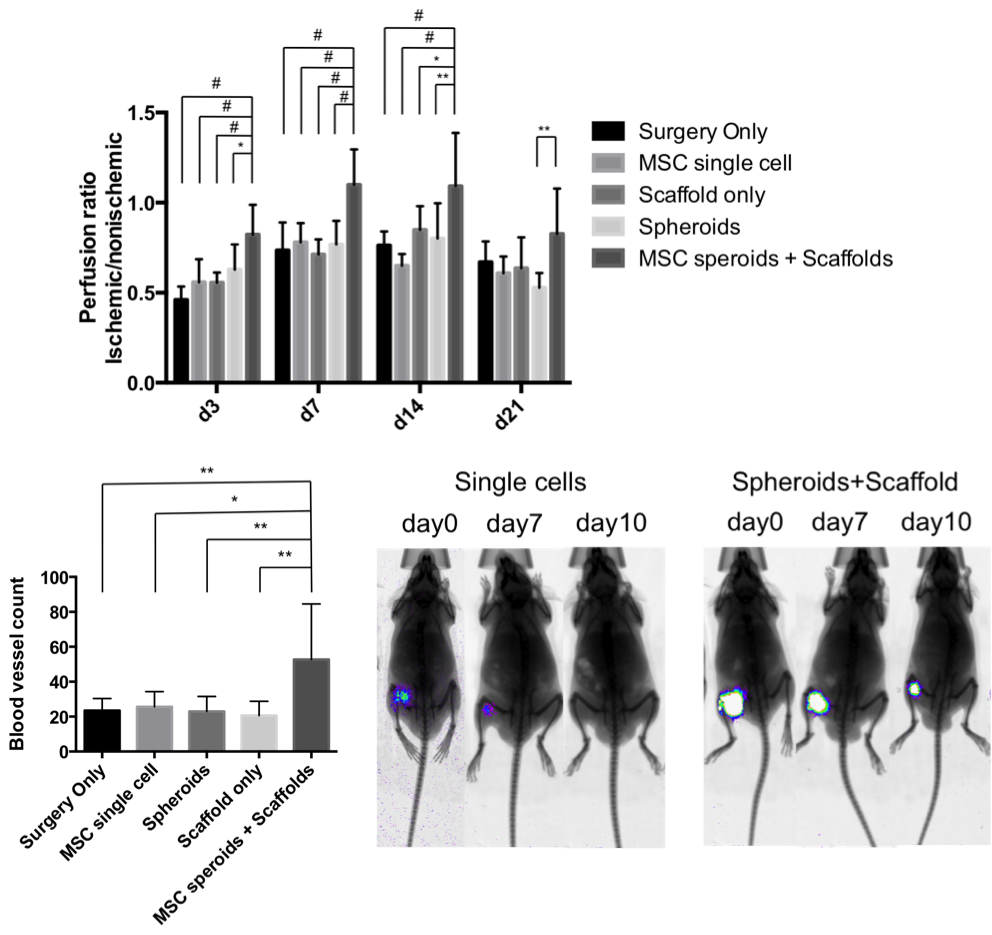
Fig. 1. A) LDPI analysis, B) blood vessel counts by lectin staining and C) representitive images from BLI.(* = p<0.05, ** = p<0.01, # = p<0.001).
Conclusion: Microwell-patterned scaffolds allow spatially controlled organization of transplanted cells. Tranplantation of MSCs on scaffolds was able to improve cell survival and functional recovery in HLI models. The approach warrants further study to understand associated mechanisms and how scaffolds may be developed to modulate injury conditions for therapeutic applications.