Introduction: Development of multifunctional nanoplatforms for cancer therapy remains a great challenge. In recent years, many nanoparticles have been developed as photothermal conversion agents for photothermal therapy, such as gold nanostars (AuNSs) and nanorods (AuNRs)[1]. However, stabilization and functional modification of nanoparticles are generally required to improve their colloidal stability, biocompatibility and specificity to the target tissues.
Materials and Methods: Folic acid (FA) was conjugated with bovine serum albumin (BSA) to form BSA-FA conjugate by activating the terminal carboxylate of FA. In the growth solution, BSA-FA conjugate stabilized AuNSs (BSA-FA-AuNSs) were synthesized through the seed-mediated method. For comparison, BSA-AuNSs without FA modification (BSA-AuNSs) were also prepared by the same method by using BSA instead of BSA-FA conjugate.
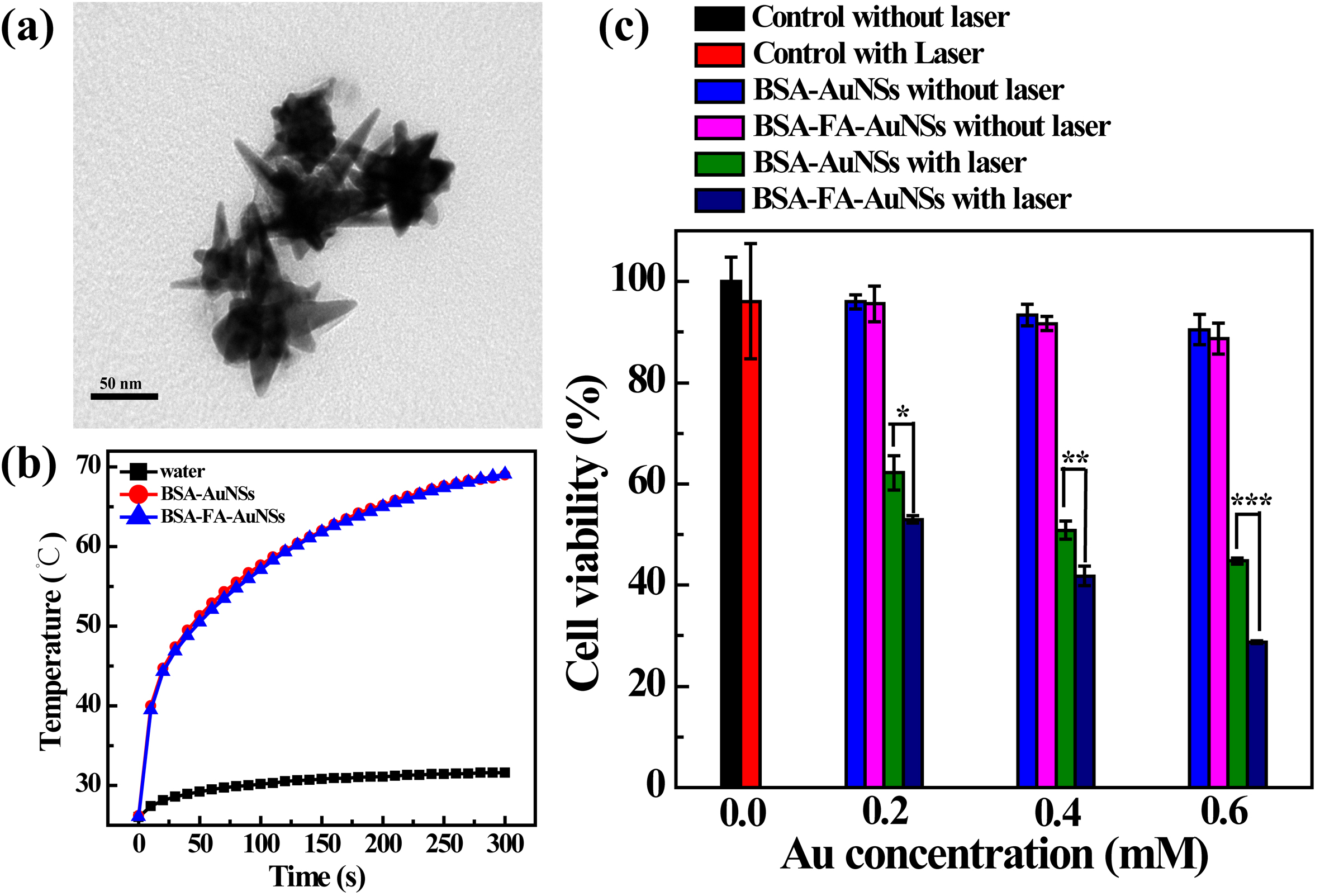
Results and Discussion: The BSA-FA-AuNSs showed star-like structure with mean size of 100.0 ± 24.4 nm confirmed by TEM (Figure 1a). The prepared NSs showed improved dispersibility and colloidal stability in water, phosphate buffered saline buffer and cell culture medium. The strong absorption property at near-infrared (NIR) regions enabled the increasing temperature of AuNSs under laser irradiation (Figure 1b). The BSA-FA-AuNSs showed not only low cytotoxicity in the range of concentrations used, but also targeting specificity to the FA receptor-overexpressed cancer cells, which was confirmed by cellular uptake. The BSA-FA-AuNSs displayed much better killing efficiency to HeLa cells under NIR irradiation compared with BSA-AuNSs without FA modification (Figure 1c).The BSA-FA-AuNSs displayed a higher photothermal conversion efficiency than other previous reported AuNSs[2]. The high affinity of BSA-FA-AuNSs to HeLa cells resulted in the higher cellular uptake and better killing efficiency.
Conclusion: BSA-FA-AuNSs was successfully prepared through a facile seed-mediated growth method for targeted photothermal ablation of cancer cells. Taking into consideration of the numerous functional groups and drug loading capacity of BSA, the BSA-FA-AuNSs may be developed into multifunctional theranostic agents for diagnosis and treatment of different cancer.
This work was supported by the World Premier International Research Center Initiative (WPI) of Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan
References:
[1] Jo H, Youn H, Lee S, Ban C. Ultra-effective photothermal therapy for prostate cancer cells using dual aptamer-modified gold nanostars. J Mater Chem B. 2014;2:4862-4867.
[2] Barbosa S, Topete A, Alatorre-Meda M, Villar-Alvarez EM, Pardo A, Alvarez-Lorenzo C, et al. Targeted Combinatorial Therapy Using Gold Nanostars as Theranostic Platforms. J Phys Chem C. 2014;118:26313-26323.