Like most cells, uterine smooth muscle cells display different differentiation and proliferation characteristics under 2-D and 3-D culture conditions. To further understand the culture behavior of uterine smooth muscle cells, we sought ways to control media flow, scaffold density, and local environmental conditions known to influence cell development. We selected hTERT immortalized myometrial smooth muscle cells (hTERT) as a cell line model for uterine smooth muscle due to the stability and consistent proliferation properties.
To control for the structural factors of the scaffold, we investigated growth on glass wool as a model. Glass wool may enhance nuclear pleomorphism of mesothelial cells [1], however we propose that glass wool offers three key advantages: 1. The known adhesion characteristics of glass; 2. Order-of-magnitude (although not precise) control over mesh dimensions; 3. Absolute structural and chemical stability over time.
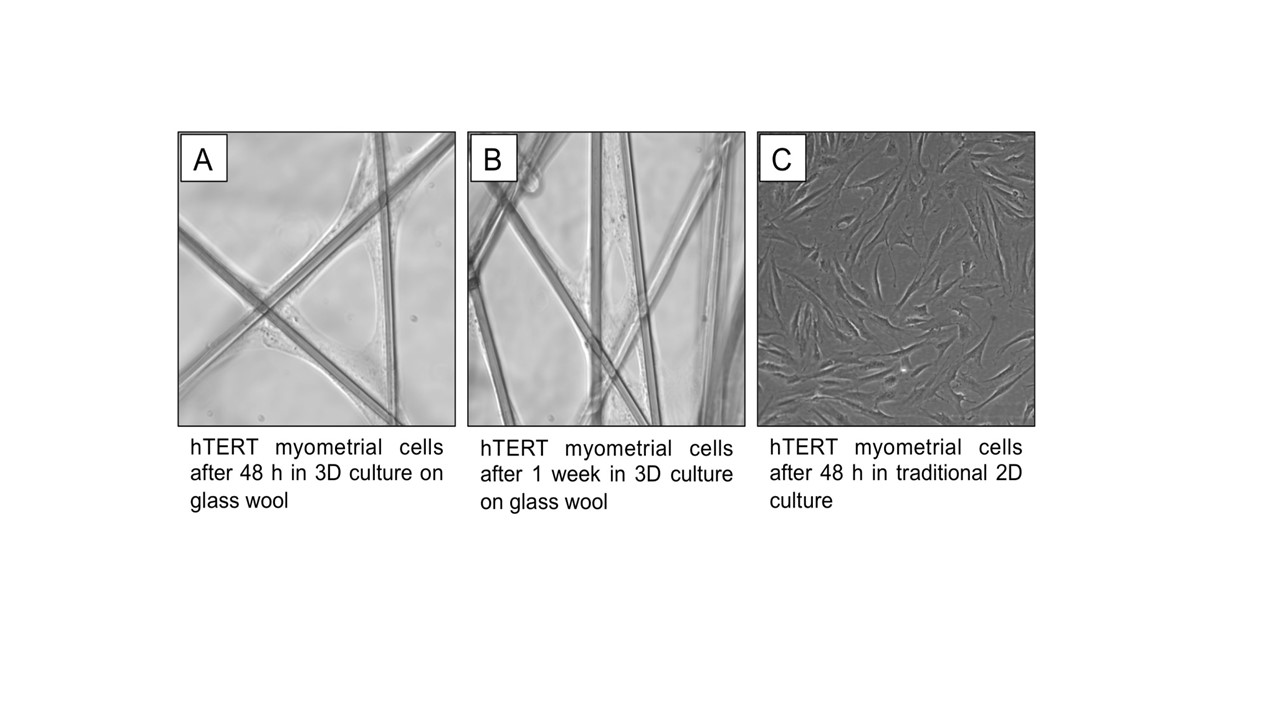
We found hTERT cells adhered and proliferated on glass wool fibers. Removal of the cells in high yield from the glass wool remains a challenge. However, on a per-cell basis, assessments of protein density, proliferation markers, and contraction-associated proteins are feasible under 3-D culture conditions designed to systematically modulate cellular proliferation/differentiation.
While glass wool will not be the ideal scaffold choice for creation of an implantable engineered tissue, it allows the study of cellular responses without the confounding effects of time-dependent variations of cell-scaffold interactions, and hence allows separation of these key experimental variables. This information will provide the basis for understanding the confounding effects of non-glass substrates in long-term 3-D culture.
References:
[1] Pelin K, Kivipensas P, Linnainmaa K. Effects of asbestos and man-made vitreous fibers on cell division in cultured human mesothelial cells in comparison to rodent cells. Environ Mol Mutagen. 1995;25(2):118-25.